Cryopreservation involves collecting ripe gametes from breeding stock and preparing them in a solution that resembles their normal internal environment, and then storing them at very low temperatures © AGGRC
Cryopreservation methods have not yet been utilised on a commercial scale in any of the major commodity species like salmon, tilapia, catfishes or carps but this may change in the coming years. Cryopreserved gametes will probably have more practical applications in hatchery settings where artificial fertilisation is already utilised, as would be the case for some salmonids, hybrid striped bass, hybrid catfishes and sturgeon to name just a few.
What is cryopreservation? Some of the descriptions provided here involve simplification, or possibly oversimplification, but this is often a necessary approach for the scope of this series.
The typical methodology, in an aquaculture setting, involves collecting ripe gametes from breeding stock and preparing them in a solution that resembles their normal internal environment in terms of osmolality, pH and other attributes. Various compounds are then added to the solution to reduce the deleterious effects of freezing and thawing. Samples are cooled to very low temperatures and subsequently stored at even colder temperatures to halt all cellular metabolic processes (usually in liquid nitrogen at -196°C). When everything goes right, samples will be biologically viable after thawing.
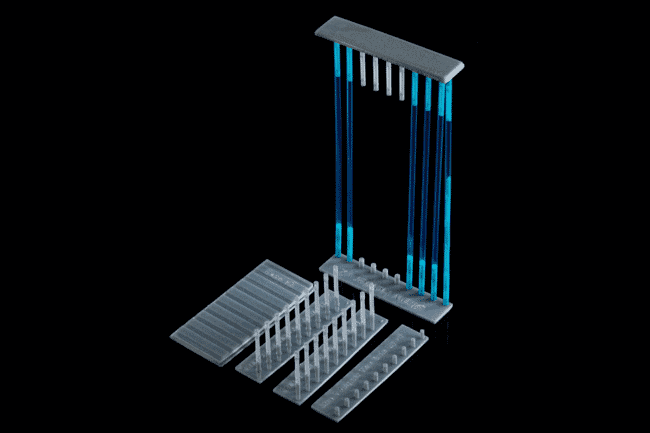
This system allows the freezing of egg strands and tissue samples, and the subsequent loading into cryopreservation straws. Reduces the handling of the sample and allows different freezing methods to be tested © AGGRC
Why do it? Using cryopreserved gametes can result in better synchronisation of reproduction in a hatchery setting, more efficient use of sperm (both in terms of quantity of offspring and maintenance of genetic variation) and easier dissemination of germplasm for selection and conservation programmes.
Cryopreservation of sperm has been achieved in many finfish species (some reports suggest well over 200), but only limited success has been reported with fish eggs or zygotes. Sperm typically lends itself to cryopreservation methods because of several inherent characteristics: permeability of the cell membrane, overall small cell volume and a simple structure lacking most internal organelles. Nonetheless, fish sperm rely on a fragile structure called the flagellum to provide locomotion, which can easily be damaged in the process.
A number of laboratories have reported success in freezing and reviving fish oocytes and even embryos in recent years, but a combination of factors has limited the use of this approach. These include the potential for egg activation upon immersion in various fluids, impermeability of egg choria with the resultant tendency for intracellular ice to form during freezing, and the presence of large amounts of yolk material. Nonetheless, some progress is being made in this area of cryopreservation research (Li et al. 2022).
Sperm cryopreservation has also been widely applied to a number of molluscs, and successful cryopreservation and thawing of developing larvae has been demonstrated in a number of bivalve species (Yang & Huo, 2022). Unfortunately, the mechanics of crustacean reproduction complicate the application of cryopreservation for these species.
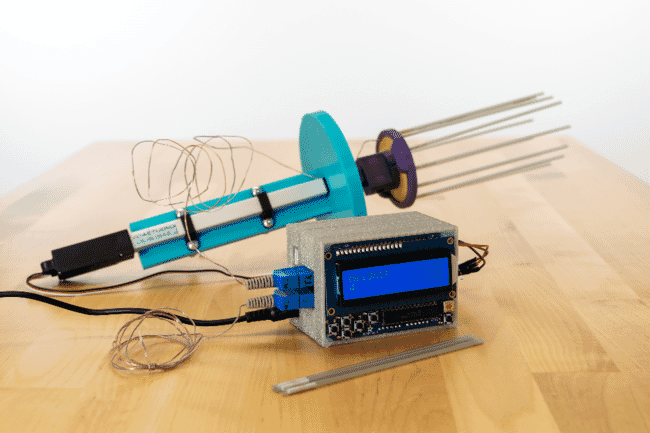
Motorised Cajun Ejector with a controller and sensor to determine the position of the samples (in the straws) © AGGRC
Most decapods of commercial importance utilise structurally complex spermatophores that are transferred from male to female, with individual non-motile sperm subsequently released during oviposition. This makes uniform, rapid freezing difficult. Although success has been reported in cryopreservation of Penaeus vannamei spermatophores (Paniagua-Chavez & Morales-Ueno 2019), other decapods have proven more problematic. Aquino et al. (2021) provide an exhaustive review of the current state of the art in cryopreservation of commercially important crustaceans.
The methodology of cryopreservation
The actual methodology used for cryopreservation of aquatic species is fiendishly complicated. Several processes are going on simultaneously when cells are cryopreserved. Water inside the cells can begin to freeze and form damaging ice crystals, especially at high rates of cooling. At lower cooling rates, water in the solution surrounding the sperm may begin to form ice crystals which results in a much more concentrated liquid over time. This tends to draw out water across the cells’ membranes, ultimately dehydrating them. Luckily, there are a number of chemicals that can be used to reduce or prevent the harmful effects of these processes. Collectively they are referred to as cryoprotectant agents (CPAs). This approach was first used when Polge et al. (1949) showed that the addition of glycerol allowed survival of fowl sperm when frozen and thawed.
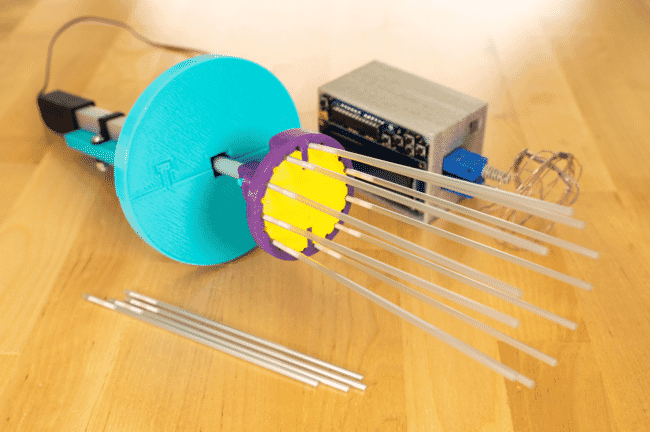
Motorised Cajun Ejector with a controller and sensor to determine the position of the samples (in the straws) © AGGRC
Two types of cryoprotectants are commonly used for aquatic species: permeating and non-permeating. Permeating CPAs can cross cell membranes to reduce internal ice crystal growth, reduce water loss from the cell and/or help stabilise salt concentrations. Examples include DMSO, methanol, propylene glycol and glycerol. Unfortunately, these compounds are often toxic at higher concentrations. This forces a trade-off between protection and toxicity.
Non-permeating protectants function in the extracellular medium. Examples include various sugars and polymers. Some protocols have included egg yolk serum, skimmed milk and even anti-freeze proteins as non-permeating CPAs. They depress the freezing point of the solution and can minimise the effects of osmotic damage and cold shock.
Sperm activation can be a problem during freezing or thawing. For most freshwater fish, activation is triggered by a reduction in osmotic pressure, while an increase in osmotic pressure starts the process for most saltwater species. This complicates freezing and thawing processes, respectively, because the build-up of external ions in solution (as ice forms) could potentially activate sperm of marine species and the decrease in ions due to melting ice during thawing could have a similar effect for those of freshwater fishes. Physical damage can also occur in sperm cells, especially to the midpiece (the power plant) and the flagellum (the propeller, as it were). Both types of damage can greatly reduce motility, resulting in low rates of fertilisation.
With so many factors involved, it’s understandable that each species exhibits distinct requirements for optimum cryoprotectants as well as the speed of both temperature reduction and subsequent thawing. These must be established through trial and error.
Virtually no standardisation of methods or equipment has taken place in the research community, and in some cases many different protocols have been used for the same species with disparate results. Differences in in diluents, cryoprotectants, freezing and thawing patterns and sperm quality all serve to increase the difficulties in building on and replicating existing research results. And, apart from differences in laboratory methodology, different populations can exhibit different sperm quality as a result of environmental conditions. At the end of the day it’s difficult to know if a cryopreservation protocol worked well or not without some evaluation of sperm quality prior to freezing – but again, these procedures are not standardised.
The tools of the trade
Researchers working in this line of inquiry are generally forced to use high-priced equipment developed for terrestrial animals as opposed to aquatic species, such as computer controlled programmable freezers, flow cytometers, freezing straws developed for livestock semen and other tools. Conversely, those without access to such resources are forced to improvise with comparatively unsophisticated materials.

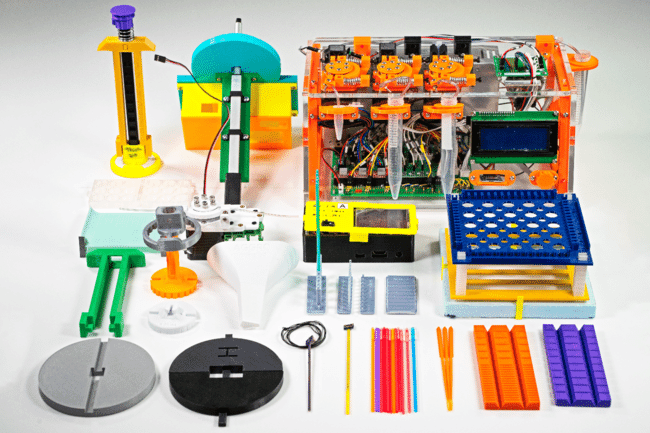
This device allows equilibrium freezing (cryoprotectant and relatively slow freezing) of genetic material at a controlled rate, to produce standardized samples, suitable for repositories. It is useful for cryopreservation in the field, or in laboratories that do not process enough samples to justify a price ticked in the tens of thousands of dollars. The cost of the manual device is below $10. A motorized device is also available. © AGGRC
One common example involves the vapour cooling method, where samples are suspended in the vapour above a reservoir of liquid nitrogen. Optimal cooling curves are based on height/time combinations in the vapour. This not only involves a trial and error approach, but is also often impossible to standardise and replicate due to varying factors such as volume of liquid nitrogen, sample positioning, buoyancy of the sample container once placed into the liquid, container sizes and thickness, and endless other considerations.
Up to now, most of the work published on cryopreservation of aquatic species has reflected multiple intellectual and biological exercises with separate, isolated protocols and little opportunity for reproducibility or standardisation. But all that is changing. A multidisciplinary group of engineers, biologists and “makers” is applying a new way of tackling the lack of affordable, customisable equipment and tools while improving standardisation of procedures. The concept of open technologies holds the promise of a community of developers, makers and users of tools, software and equipment specifically focused on advancing the science of aquatic cryopreservation.
A team of researchers at the Lousiana State University’s AgCenter’s Aquatic Germplasm and Genetic Resources Center (AGGRC) has begun an ”open hardware” approach that incorporates accessible technologies such as 3-D printing, microprocessors, open-access electronic peripheral components, internet connectivity and open-licence design software to develop specialised tools for the challenges facing cryopreservation researchers working with aquatic species (Liu et al. 2021). Especially those working with tight budgets. As the resulting user community expands, opportunities to distribute, share, modify and improve these cryopreservation tools will also expand. Such a community could also foster combined aggregate development of germplasm repositories.

This device reduces the cost of freezing genetic material at a controlled rate. The resulting samples can be stored in a dewar. The position of the freezing platform (dark blue) from the frame base determines the freezing rate. The device is used floating in liquid nitrogen in a foam box. © AGGRC
Ensuring access
The key to an open technology strategy is access, which in turn allows community collaboration. The necessary access channels are already used by most of us on a daily basis, in the form of websites, shared servers, on-line videos and data sharing applications. Some examples of open platforms already used by aquaculture researchers include the International Aquaculture Feed Formulation Database and GenBank. With collaboration channels established, open-licence computer-aided design and manufacturing software can be used anywhere with 3-D printers, compact computer controlled mills and 3-D scanners, to produce components for all sorts of in-house hardware needs.
One example, developed by the AGGRC team, is a shipping dewar positional cooling device (SDPCD) for freezing and storing samples. At a cost of roughly $8, the device includes eight 3-D printed components and a commercially available metal spring. The SDPCD allows for standardised cooling rates ranging from 1 to 68 °C per minute, allowing researchers in different locations to better harmonise their cooling methodology. Other devices have been designed by the team to allow for standardisation of cooling rates when using polystyrene boxes to hold the liquid nitrogen.
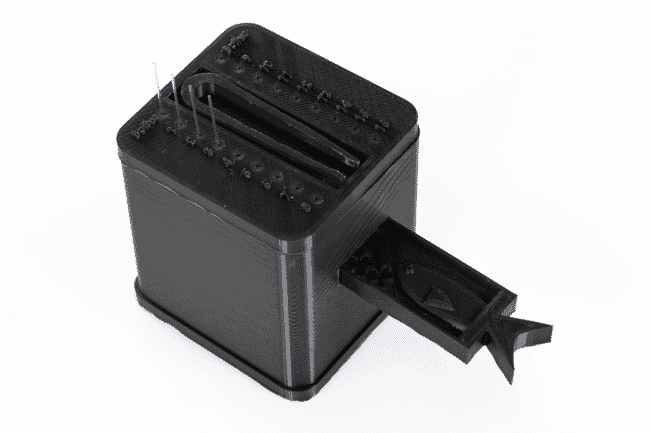
A device produced by mechanical engineering students at Boston University, under the direction of AGGRC researchers. © AGGRC
Another interesting concept developed by the AGGRC is a controlled cooling conveyor device (CCCD) that moves samples through a cooling vapour environment and automatically drops them into the liquid nitrogen below upon reaching the appropriate temperature. Low cost components can even be configured to allow for temperature monitoring inside freezing straws.
The ongoing collaborations at the AGGRC and beyond illustrate the opportunities, tools and resources that are available all around us – not just for cryopreservation, but also for numerous other aspects of aquaculture research as well. When researchers and end users are working together as a community, the possibilities can be endless.








