
© Eric Boulommie
Conserving genetic resources for endangered aquatic species has taken on increasing importance in recent years. As a result of advances in the science of cryopreservation it has become fairly easy to freeze sperm of many aquatic species, but mature ova and developing embryos are another story, at least for fishes.
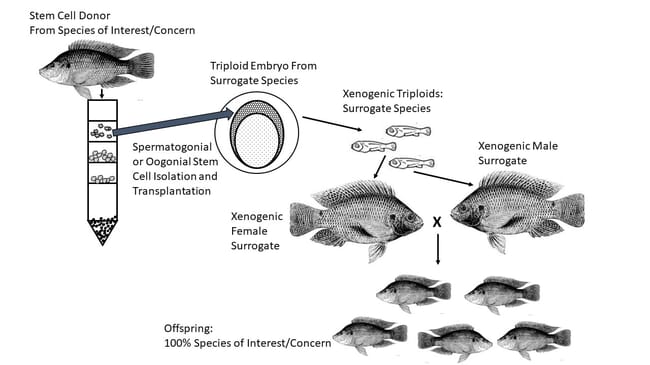
Their comparatively large size, high yolk content and limited membrane permeability usually make them difficult or impossible to cryopreserve, but in recent years a viable workaround has been demonstrated. The tissues and/or cells that ultimately develop into fish eggs can be cryopreserved, subsequently thawed, and transplanted into sterile recipient fish. These surrogate recipients can be from the same species, related species, or in some cases completely unrelated ones. When everything works, the recipient fish develop functional male or female gametes with genetic material from the original donor. As a result, this approach offers distinct advantages for conservation of threatened or endangered species.
The science (simplified)
These new frontiers in aquatic conservation are focused on the use of germ cells and gonadal stem cells, with the latter emerging as the material of choice when working with fish. Without going into excessive detail, stem cells are basically undifferentiated cells that can multiply into many more cells of the same type, remain dormant, or differentiate to take on specific functions. Different tissues and organs, including gonadal tissues in fishes, typically contain some stem cells that can develop into new, differentiated cells. To briefly summarise the origins of those gonadal tissues, very early in the development of fish embryos two fundamental cell types can be distinguished: germ cells and somatic cells. As the somatic cells begin to form the entirety of the organism, the germ cells find their way to the tissues that will become ovaries or testes and begin to proliferate.
If gonadal stem cells (or suitable spermatogonial or oogonial tissues) from donor fish can be cryopreserved and successfully recovered, they can be introduced into surrogate individuals where they will function just like germ cells, migrating to the appropriate internal location and developing into functional gonads (Yoshizaki and Lee 2018). Recipient fish are made sterile beforehand, either through induction of triploidy or suppression of native gonadal cells. And, in many cases the recipients can be from distinctly different species. When the recipients are a different species than the donor, even if closely related, they are referred to as “xenogens.” A number of recent research results confirm this approach represents a powerful new tool for preservation of genetic resources in a number of fish species.
Does it really work?
In 2010, Lacerda et al. reported on efforts to conduct spermatogonial stem cell transplantation in tilapia. They transplanted fresh or cryopreserved stem cells from one strain of tilapia into previously sterilised males of another strain. The cryopreserved stem cells developed into spermatozoa in the recipient fish, with a colonisation rate of 88 percent. The recipient males ultimately produced viable spermatozoa and sired progeny with the donor strain genotype.
That same year, Yoshizaki et al. demonstrated that when transplanted into newly hatched fry, rainbow trout ovarian germ cells eventually differentiated into eggs in female recipients and sperm in male recipients. Similar results have been reported in other fishes, and this recipient-based plasticity might also provide an alternative approach for monosex culture in a number of species.
© Greg Lutz
In 2012 Lee et al. were able to transplant previously frozen spermatagonia into triploid rainbow trout hatchlings. Nearly half of these fish went on to produce functional eggs or sperm, depending on their phenotypic sex, which when combined resulted in normal offspring. Similarly, in 2016, Lee and Yoshizaki demonstrated the cryopreservation, thawing and transplantation of testicular cells of the endangered Manchurian trout into recipient triploid hatchlings of the same species. Transplanted spermatogonia migrated and were incorporated into the triploids’ gonadal tissue, with some recipients developing maturing testes while others produced maturing ovaries. On a cautionary note, under real world conditions these approaches would require a number of stem cell donors in order to minimise inbreeding impacts, with xenogens from one donor only being spawned with those from other donors.
Going a step farther with another endangered fish, Psenicka et al. (2016) used early-stage testicular and ovarian tissue and cells from Siberian sturgeon to evaluate various handling and freezing protocols. Both whole tissue and dissociated cells exhibited good survival after thawing. When transplanted into sterlet larvae, after 90 days they had proliferated in more than half of the recipients. And further pushing the boundaries of phylogenetic distance between donors and recipients, Silva et al. (2016) successfully transplanted spermatogonial stem cells from Jundia catfish (Rhamdia quelen) to male Nile tilapia. Male tilapia were sterilised prior to transplantation using high temperatures and bisulfan injections. By 120 days post-procedure all the recipient fish had begun producing sperm.
Common carp are one of the most widely cultured species in inland waters and over hundreds of years a number of distinct strains have been developed. Concerns have been raised that some of these “heirloom” varieties could be lost if not conserved. Working with oogonial cells, Franek et al. (2019) provided a thorough review of cryopreservation procedures to preserve genetic resources in this species. Interestingly, immature donors exhibiting early stages of ovarian development provided the best cryopreservation results, probably due to their cells still having adequate levels of membrane permeability. Yaraş and Çek-Yalniz (2021) also reported higher success when isolating oogonial stem cells from brown trout juveniles, roughly 15 cm in length.
More recently, Ye et al. (2020) developed a procedure for cryopreservation of American paddlefish and Yangtze sturgeon gonadal stem cells. After one year in cryopreserved storage, these cells were thawed and transplanted into sturgeon larvae, where they were successfully incorporated. These findings, along with those of Psenicka et al. (2016), are especially encouraging when one considers that roughly 80 percent of the surviving species of sturgeons and paddlefish across the globe are considered endangered or critically endangered by the International Union for the Conservation of Nature.

© Dr Chris Green
Needle immersed vitrification
There are thousands of distinct lines of zebrafish in biomedical research, with more being developed each month. With the eventual goal of more efficient conservation of zebrafish germplasm, Marinović et al. (2018) presented a viable method for cryopreservation of early stage zebrafish germ cells using spermatogonia and needle immersed vitrification (NIV). In a follow up study the group presented more NIV results with high success rates. NIV offers some distinct advantages when cryopreserving small tissue samples. The cryoprotectant requirements are reduced because tissue samples are pinned on an acupuncture needle, placed in cryoprotective media then removed and placed in a vitrification solution, and subsequently plunged directly into liquid nitrogen.
Several of the same researchers from the Marinović et al. studies mentioned above had already reported on the optimization of NIV protocols for salmonid ovarian tissue in 2017, specifically juvenile ovarian tissue from brown trout. And in 2022 some of the same research group reported on the use of NIV using ovarian tissue in the sturgeons Acipenser ruthenus and A. gueldenstaedtii. Thawed cells successfully colonised the gonads of recipient fish, and the results confirmed NIV as an additional tool for conservation of endangered sturgeon species.
Production aquaculture
Apart from conservation initiatives, the use of surrogate broodstock offers a number of potential benefits for genetic improvement in aquaculture. Fish with superior or unique traits can supply cells for transplantation into multiple recipients, vastly enhancing the production of selected offspring. It may also be possible to utilise recipient species with relatively short generation intervals to accelerate selection programmes for donor species that require longer times to reach maturity. And, when hybrid production stocks are desired, xenogeneic technologies may offer a practical alternative to large-scale artificial spawning.
In the US, catfish aquaculture has become largely reliant on hybrid stocks produced using blue catfish (Ictalurus furcatus) males and channel catfish (I. punctatus) females. These hybrids offer superior characteristics in terms of growth, feed conversion and disease resistance. Unfortunately, they must be artificially produced in hatcheries due to behavioural incompatibilities that result in limited and largely unreliable volitional spawning between the parental species. And, since blue catfish males cannot be strip-spawned, they must be sacrificed in the process, significantly increasing annual requirements for male broodstock. Researchers at Auburn University began evaluating the use of xenogeneic methods to address this situation a number of years ago, and their progress has been impressive.

© Dr Chris Green
Perera et al. (2017) published the first report of xenogeneic production of channel x blue hybrids, albeit with very limited results. They transplanted blue catfish spermatogonia into triploid male channel catfish, and two years later they collected sperm from one of these xenogens and successfully used it to fertilise channel catfish eggs, resulting in hybrid offspring. Shortly thereafter Shang et al. reported on the isolation and successful transplantation of blue catfish testicular germ cells into channel catfish blastulae. The resultant xenogeneic channel catfish males would, in theory, be able to mate naturally with channel catfish females every year after reaching maturity, producing hybrid offspring in commercial quantities.
By 2020, Abualreesh et al. developed and published a reliable protocol for freezing blue catfish spermatogonia, with additional refinements
in 2021. Realising that having frozen stem cells in repositories would have real potential to advance commercial scale production of hybrid catfish, the Auburn researchers pressed on and by 2022 Hettiarachchi et al. demonstrated the production of channel catfish xenogens using cryopreserved blue catfish testicular and ovarian tissues.
This research continues at Auburn, with methods and protocols advancing continuously, but some new applications may also be emerging. One typically does not consider fish eggs to be an especially valuable commodity, with the exception of caviar. Especially sturgeon caviar. In 2023 Jacob Al-Armanazi completed his master’s thesis at Auburn. One focus of his research involved transplanting oogonial and spermatogonial stem cells from lake sturgeon into triploid hybrid catfish (white catfish x blue catfish) and from Siberian sturgeon into triploid channel catfish. Almost 89 percent of the recipient hybrid catfish exhibited lake sturgeon stem cell proliferation, and although results were not as impressive for transplanted channel catfish, Siberian sturgeon stem cells persisted in almost 77 percent of recipients.
To summarise, as our understanding and mastery of xenogeny production in fish advances, a number of benefits may result. Conservation and preservation of germplasm will continue to be a significant concern throughout the world, for all those working with threatened and endangered fish species and also for a number of sectors within commercial aquaculture. In the coming years cryopreservation and subsequent transplantation of gonadal stem cells will take on increasing importance in a number of fish species.








