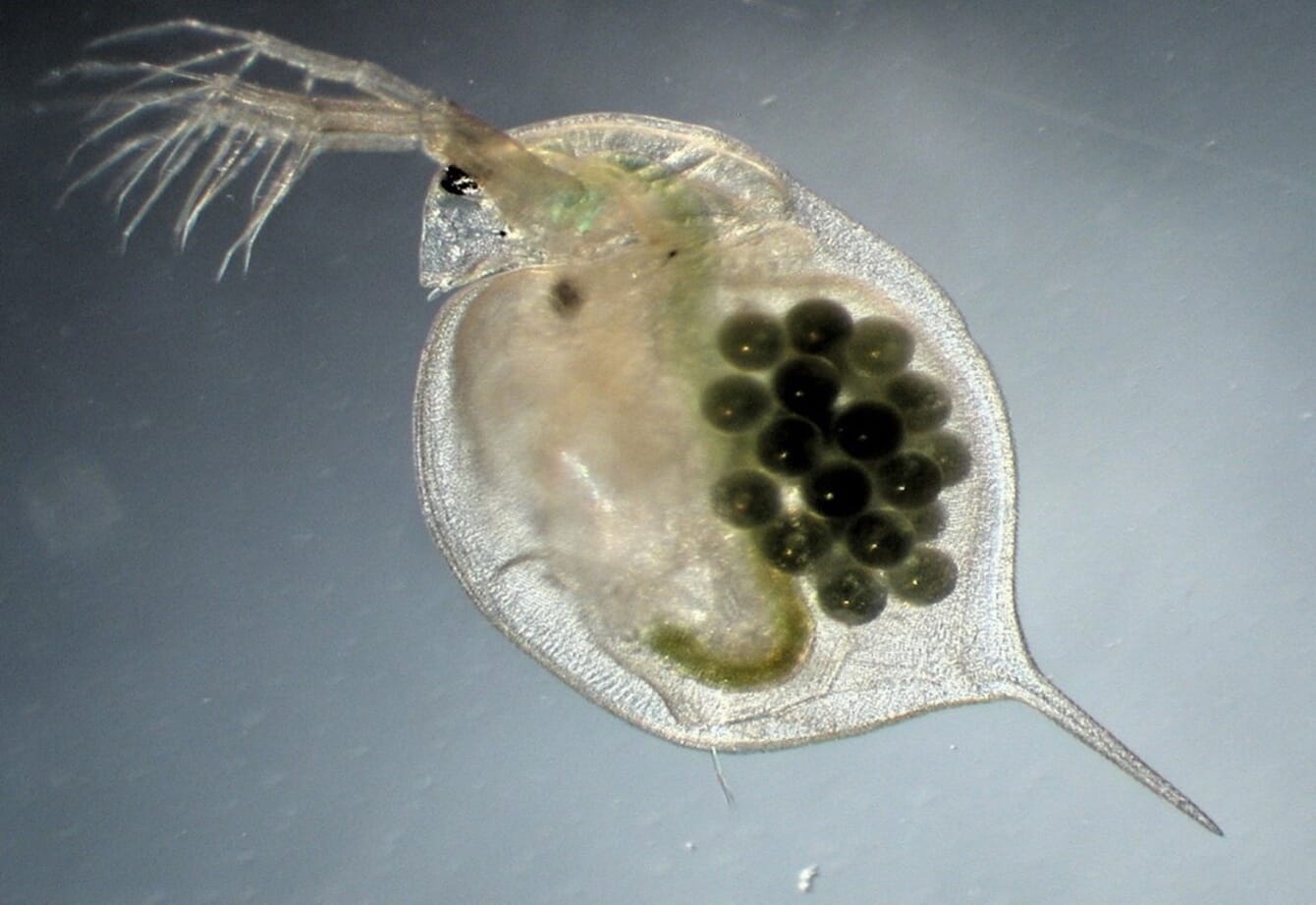
Researchers discovered that after algae had been harvested by freshwater zooplankton D. magna, all phosphorous and 90 percent of excess nitrogen had been assimilated by the water fleas
Just as the waste nutrients of terrestrial fertilisers can stimulate harmful blooms of microalgae when they wash into aquatic environments, the nutrient-rich wastewater from open-system aquaculture operations can stimulate similar harmful eutrophication events, when not properly managed. The increasing implementation of indoor recirculating aquaculture systems (RASs) has been used as a solution to mitigate this issue for both marine and freshwater aquaculture. But it’s challenging to remove the excess nutrients – which would harm cultured fish – from the water before recirculation.
Whilst RASs can effectively eliminate environmental and public health concerns over farm nutrient emissions into the aquatic environment, the quality of recirculated water must be precise in order to maintain fish health. This problem has been addressed in the past by using biofilters – living microbial colonies that remove excess nutrients, such as nitrogen and phosphorous – however, an interdisciplinary research team at the Technical University of Denmark has shown that the sustainability of these systems can be taken one step further.

Using the algae, the researchers extracted 100 percent of the phosphorous and 70 percent of the nitrogen from recirculated water sourced from a freshwater RAS operation © Dorottya Sarolta Wagner-Zafirov
The team used a combined approach of using microalgae to bioremediate the recirculated water, and bio-harvesting of these algae by zooplankton to maximise resource recovery, with the zooplankton being a suitable live feed source, particularly for fish larvae.
Like terrestrial plants, marine microalgae require nutrients such as phosphorous and nitrogen – two of the most significant nutrient emissions from finfish aquaculture – to grow, and researchers have exploited this trait to remove excess nutrients from recirculated, nutrient-rich water. Using the algae species Chlorella vulgaris and Scenedesmus dimorphis, the researchers managed to extract 100 percent of the phosphorous and 70 percent of the nitrogen from recirculated water sourced from a freshwater RAS operation.
Although the use of microalgae to extract excess nutrients from RAS facilities is not a novel technique, the challenge of this approach lies in the harvesting of the algae after growth. The researchers overcame this challenge, however, simply by exploiting the natural behaviour of the zooplankton species Daphnia magna – the humble water flea, which graze on freshwater algal communities. After harvesting of the algae by D. magna, all phosphorous and 90 percent of excess nitrogen had been assimilated by the water fleas, showing promise of this approach for efficient nutrient removal prior to water recirculation, and the potential for commercialisation of this approach.

“To harvest the Daphnia we just used sieves, and we could even use this method to separate adults from neonates – this is the best approach. This is much easier than harvesting algae, which often requires more time, energy and resources,” explains Professor Borja Valverde-Perez, the corresponding author of the study.
The researchers have proposed that the D. magna populations fed on experimental diets of microalgae grown on recirculated water may provide a suitable feed substitute for fish meal, with the fleas having a suitable protein content of 30 percent dry weight, and favourable fatty and amino acid profiles, including high levels of lysine and methionine, which are often the most limiting of the essential amino acids.
The researchers believe that D. magna fed on RAS-grown microalgae would be a suitable feed for omnivorous fish and shrimp. Not only would this feed source have the potential to reduce costs to aquaculturists on a commercial scale, but would also reduce pressure on wild fish stocks, which are a key source of fish meals used in commercial feeds.
“I understand it is a good live feed, and is better than using the algae itself as a feed, due to better palatability and digestibility. As a live feed, Daphnids are particularly good for fish larvae, as the fish have to swim to catch them, which keeps the fish larvae active,” says Professor Valverde-Perez.
When asked for predictions of the Daphnid biomass that an aquaculture site may be expected to produce, Professor Valverde-Perez said: “We used wastewater effluent from a site that was not that polluted, and as such we did have a bit of trouble growing the algae optimally. In real cases, I expect Daphnia productivity would be higher compared to our study.”
This suggests that, although it may not be possible to extrapolate the potential Daphnid productivity of an RAS facility from this data, it could be expected that the harvested Daphnid biomass would be sufficient to provide a significant source of live feed.
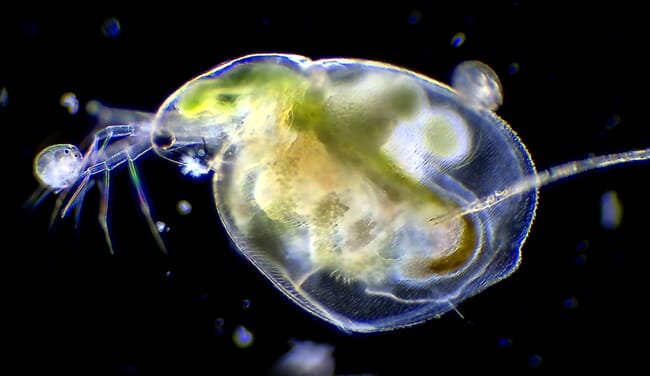
The study suggests that any harvested Daphnid biomass would be sufficient to provide a significant source of live feed © Shutterstock
However, whilst the prospect of reduced costs and decreased environmental impacts of feeds may be exciting, many questions need to be answered before this approach to recirculating aquaculture can be upscaled and commercialised.
Microalgae, for example, can accumulate toxic compounds from their immediate environment, and these can then be transferred to higher trophic levels, and so the potential for feeds sourced in this manner to result in accumulation of toxic compounds in aquaculture products must be investigated.
Additionally, whilst this research supported the potential for microalgae to remediate water from recirculating aquaculture systems, only nitrogen and phosphorous levels were measured, and the full nutrient profile of recirculated water must be considered to ensure safety of animals contained within the system.
Finally, although this study used Daphnia magna, a freshwater zooplankton, to harvest algae from a freshwater RAS, the researchers say that a similar approach can be adopted in marine RAS facilities, where copepods can be used in place of D. magna.




