Identity
Ruditapes philippinarum Adams & Reeve, 1850 [Veneridae]
FAO Names: En - Japanese Carpet Shell, Fr - Palourde Japonaise, Es - Almeja Japonesa

Biological Features
Shell solid, equivalve; inequilateral, beaks in the anterior half; somewhat broadly oval in outline. Ligament inset, not concealed, a thick brown elliptical arched body extending almost half-way back to the posterior margin. Lunule elongate heart-shaped, clear though not particulary well defined, with light and dark brown fine radiating ridges. Escutcheon reduced to a mere border of the posterior region of the ligament. Sculpture of radiating ribs and concentric grooves, the latter becoming particulary sharp over the anterior and posterior parts of the shell, making the surface pronunced decussate. Growth stages clear. Three cardinal teeth in each valve; centre tooth in left valve and centre and posterior in right, bifid. No lateral teeth. Pallial sinus relatively deep though not extending beyond the centre of the shell; it leaves a wedge-shaped space between its lower limb and the pallial line. Margin smooth. Extremely variable in colour and pattern, white, yellow or light brown, sometimes with rays, steaks, blotches or zig-zags of a darker brown, slightly polished; inside of shell polished white with an orange tint, sometimes with purple over a wide area below the umbones.
View SIDP Species fact sheet
Images Gallery

Profile
Historical Background
The Japanese carpet shell (also known as the small-neck or Manila clam) is a subtropical to low boreal species of the western Pacific and is distributed in temperate areas in Europe. Wild populations are found in the Philippines, South and East China Seas, Yellow Sea, Sea of Japan, Sea of Okhotsk, and around Southern Kuril Islands. Its culture was initiated in those areas from the initial traditional fishing activities by the collection of wild seeds.
Of considerable commercial value, Japanese carpet shells have been introduced to several parts of the world, where they have become permanently established. Accidentally introduced during the 1930s to the Pacific coast of North America along with Pacific cupped oyster seed, Japanese carpet shells have spread to colonize the coast from California to British Columbia. Besides public fisheries, hatchery production has facilitated Japanese carpet shell culture along the Pacific coastline. Japanese carpet shells were also transferred from Japan to Hawaiian waters early in the 20th century, where wild populations now occur. Overfishing and irregular yields of the native (European) grooved carpet shell, Ruditapes decussatus, led to imports of R. philippinarum into European waters. This species was introduced in 1972 through French hatchery production. Additional imports into the United Kingdom from Oregon (United States of America) were followed by numerous transfers within European waters for aquaculture purposes (Portugal, Ireland, Spain, and Italy). Moreover, aquaculture trials resulted in seed being imported into French Polynesia, the US Virgin Islands, Norway, Germany, Belgium, Tunisia, Morocco, and Israel. Following the large aquaculture hatchery based on developments in Europe over the 1980s, natural reproduction resulted in a geographical expansion of wild populations, particularly in Italy, France, and Ireland, where Japanese carpet shells have proved to be hardier and faster growing than the endemic R. decussatus. Consequently, R. philippinarum populations are now the major contributor to clam landings in Europe, and are the focus of intensive public fisheries, competing with aquaculture products in several rearing areas.
Main Producer Countries
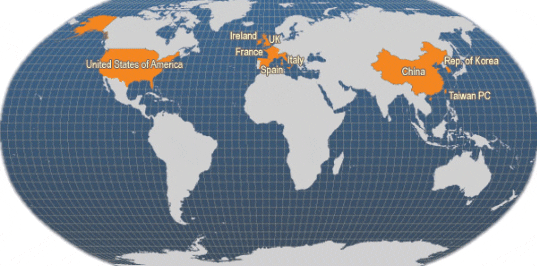
Main producer countries of Ruditapes philippinarum (FAO Fishery Statistics, 2006)
Habitat and Biology
The Japanese carpet shell (Ruditapes philippinarum) is native to Japan with a wide distribution in the Indian and Pacific Oceans from Pakistan to the Russian Federation (Kuril Islands). It has subsequently been introduced along the North American Pacific coast, the Hawaiian Isles and, over the last 20 years, along the European coastline from the United Kingdom to the Mediterranean Basin.
Japanese carpet shells are strictly gonochoric and their gonads are represented by a diffused tissue closely linked to the digestive system. The period of reproduction varies, according to the geographical area; spawning usually occurs between 20-25 °C. A period of sexual rest is observed from late autumn to early winter. Gametogenesis in the wild lasts 2-5 months, followed by the spawning. A second spawning event may occur in the same season, 2-3 months later. The pre-winter recovery phase facilitates energy build up, by filtering seawater still rich in organic matter and phytoplankton. Temperature and feeding are the two main parameters affecting gametogenesis, which can be initiated at 8-10 °C and is accelerated by rising seawater temperature. Its duration decreases from 5 to 2 months between 14 and 24 °C. Within this temperature range, Japanese carpet shells are ready to spawn. Although the optimal temperature is between 20 and 22 °C, 12 °C is the minimum threshold below which this species cannot spawn efficiently. Food availability influences the amount of gametes produced. Larval development lasts 2 to 4 weeks before spatfall. Settlement size is between 190 and 235 µm in shell length. Many external factors regulate spatfall success in the wild, such as temperature, salinity and currents. Larval movement mainly depends on wind driven and tidal currents. Adding pea gravel and small rocks can facilitate species recruitment in natural setting areas. The larvae settle by attaching a byssus to a pebble or piece of shell.
Production
Production Cycle
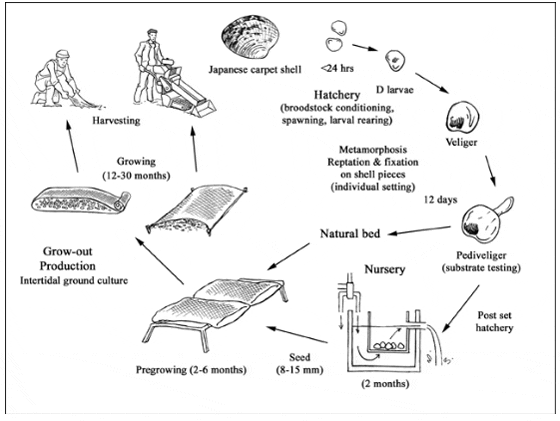
Production cycle of Ruditapes philippinarum
Production systems
The production cycle begins with the reproductive stage and seed production. Seed can be obtained either by gathering wild spat or by hatchery production. Most commercial Japanese carpet shell culture is based on intertidal on-bottom culture using wild seed or hatchery products (2-3 mm size).
Seed Supply
Natural spat supply
Together with the colourful clam Ruditapes variegata, R. philippinarum is one of the two predominant and traditional species in China, where it is mostly cultured using natural seed collected from muddy beach sand areas. In this country, seed collection involves the selection and preparation of seed collection beds, eradication of predators, and routine maintenance. Techniques have been developed to increase seed supply: these consist of shallow ponds (up to several ha in area) made in the lower intertidal area, from which all competitors, seaweed and predators are removed and the bottom smoothed. These ponds can be inoculated with Chaetoceros, a suitable food for larvae and postlarvae. Each pond can be used for rearing 2-3 batches/yr. About 75 to 150 million seed clams (0.5 cm) can be grown per ha. These clams grow up to 1.5 cm by May the following year and are then replanted at 1.8 million/ha for another year to reach a 3.5 cm marketable size (19-45 tonnes/ha).
In European waters, spat supply is based both upon hatchery products as well as harvested wild seed. The availability of wild seed is a result of the invasiveness pattern of this species in areas where it was formerly cultured using hatchery-produced spat. In contrast, most of the seed supply in North America is based upon hatchery production.
Hatchery Production
The main operations consist of conditioning the breeding stock to facilitate gametogenesis; spawning and larval rearing to metamorphosis; and growing the resulting juveniles to a suitable size, either in nurseries or directly in the sea.
Broodstock conditioning takes 30-40 days at 20 °C. Spawning is induced either by thermal shock stimulus or by adding drops of sperm, or by stripping. Fertilized eggs are filtered through a 40 µm sieve and maintained in 10 litre containers until the veliger stage. Larvae are then collected by sieving and distributed into containers at a 3 000/l. Larvae are fed everyday for the first week at 30 cells/µl, then every second day until larval metamorphosis is reached (2 weeks). Salinity for breeding and rearing must be between 24 and 35‰ (tolerance range 13.5-35‰). A 15-28 °C temperature range is optimal for growth, although the species can survive at 0 ° and 35 °C for short periods of time. Pediveliger clam larvae can be prepared for shipping at this stage following a screening and counting process, and then deployment into shipping material (nytex, paper coffee filter), wrapped in several layers of moist towelling. Then, this is placed in an insulated shipping box along with an ice pack (without contact with the larvae). Alternatively, pediveliger larvae are settled in setting tanks or micronursery trays using nylon screens and a flow-through recirculating system with frequent water exchange (e.g., water tables, downwellers). Anytime the clams are removed from the water, care must be taken to ensure they do not dry out or become too warm.
Hatchery rearing requires the production of suitable microalgal species as food. Usually, the phytoplankton species used are flagellates such as Isochrysis galbana, Pavlova lutherii and Tetraselmis suecica or Platymonas sp. Combinations of flagellates with diatoms such as Skeletonema costatum, Chaetoceros calcitrans, C. gracilis and Thalassiosira pseudonana provide a well balanced diet, facilitating gametogenesis and larval development. Food quantity depends on larval density.
Nursery
Although clams have a protective shell, the shells will break if not handled with care during the screening and sorting processes.
A nursery system using an up-welling approach may be used for pre-growing seed up to 10-15 mm shell length. Slightly larger clams (6-7 mm) at 3 000/m² are deployed on the seabed enclosed in 4 mm mesh bags (1.5 m x 2 m). Other culture practices for pre-growing include placing 4-5 mm seed at a density of 10 000/m² in wooden frames covered by plastic netting, stacked underwater (in Italy) or in mesh covered wooden frames, or in mesh bags on trestles around the low spring tide area (in Ireland). Stocking densities are progressively thinned with increased growth. During the nursery stage, grow-out facilities must be cleaned, clams graded and predators such as crabs, removed. Although tray culture can be used for the early growing stage, clams grow better in the soil. Floating upwellers such as FLUPSYS (a rack structure which supports a series of containers along a central channel from where the water is forced either by using a propeller or a paddlewheel) are in use mostly in North America. Clams should be size graded to ensure that all animals are of similar size; otherwise, larger clams can out-compete the smaller ones, leading to retarded growth.
Ongrowing Techniques
At about 10-15 mm shell length, clams are ready for seeding in the substrate. The ongrowing operation is best established on intertidal sites sheltered from extreme wind, wave and tidal action. Alternatively, 400 m² oyster ponds can be used to rear clams up to the market size. Suitable substrates for on-growing sites usually consist of gravel, sand, mud and shell. Although Japanese carpet shells clam will survive in a variety of soils, a too soft bottom limits access and the type of equipment used for planting, husbandry and harvesting. Prior to seeding, the area must be properly prepared and predators removed. The plot system of cultivation uses strips of mesh, deployed over the seeded clams and ploughed in along the edges of the plot to limit crab and bird predation.
Growth and survival are directly related to stocking density. Clams (10-15 mm) are seeded at 200-300/m² and simultaneously covered with 4 mm aperture netting (1.5 wide, 300 m long) to protect them from excessive predation. In Europe, a planting machine has been developed that simultaneously ploughs in the netting and sows the seed. Deflector plates backfill the trenches, burying the edges of the net. Nets should be cleaned to avoid fouling organisms and siltation, and monitored for predators. Depending on local carrying capacity, clams will grow up to 40 mm in about 2 to 3 years.
In China, Japanese carpet shell seeds (5-10 mm) are planted at 35 million/ha, although density is based upon seed size and sediment type. Usually, clam beds are not protected with nets.
Harvesting Techniques
In China, clams are harvested after 10-16 months, at 30 mm or larger. In European waters and in Northern America, clams are usually harvested after 16 to 30 months at a larger size (30-40 mm shell length) to obtain an improved exchange value. In both cases, they are usually either raked or mechanically harvested. In Europe, manual harvesting of Japanese carpet shells consists of raking them out of the substrate and bringing them to the surface. Mechanical harvesting is carried out by suction or elevator dredges; a tractor equipped with a lateral conveyor belt can dig and grade clams from sandy bottom areas, covering more than 200 m²/hr (>600 kg/hr).
Handling and Processing
Once harvested, the clams are stored in boxes or bags and transported for mechanical grading. Then they are usually wet stored to purge grit and sand before processing and marketing. They can be wet stored longer in the low intertidal areas using plastic mesh bags, or in ponds, or suspended from wet storage rafts until they are sold.
Production Costs
Production costs are highly variable due to several factors. Depending on site characteristics, clams may take 2 to 4 years to reach marketable size, therefore affecting overall yield. Local carrying capacity, bottom softness, and the cost of operational procedures such as preparing seeding plots, removing predators, cleaning to avoid the clogging of structures by mud or algae, regular grading and sorting are among the main driving factors. In addition, the chosen rearing strategy will directly affect production costs. The size at which to purchase seeds from hatchery-nursery is critical. The lower cost of buying small seed may be balanced by increased mortality rates and higher operating costs (nursery structures); in contrast, buying larger seed is more expensive and requires higher survival rates to be cost effective. Bottom type characteristics (sandy versus muddy bottom) affect harvesting procedures and efficiency; for example, mechanical harvesting is easier in sandy bottoms, providing improved yield. All these factors should be carefully considered when planning clam culture in order to optimize production costs and select and appropriate rearing strategy.
Diseases and Control Measures
| DISEASE | AGENT | TYPE | SYNDROME | MEASURES |
| Viral | Herpes-like virus | Virus | Sporadic high mortalities in hatcheries | No curative measure. Prevention & site selection; monitoring clam transfer |
| Rickettsial | Rickettsiosis | Intracytoplasmic microcolonies | Microcolonies occur in the epithelial cells of gills & digestive gland; infections usually light & not associated with disease; no macroscopic effect | No curative measure. Prevention and site selection |
| Bacterial (abnormal calcification; brown ring) | Vibrio tapetis | Bacteria | Stunted growth; brownish coloured deposit along leading edge of mantle caused by organic material (conchiolin) | No curative measure. Prevention & site selection; monitoring clam transfer |
| Protozoan parasite | Perkinsus-like organisms and Perkinsus atlanticus | Protozoan parasite | Greyish-white nodules or cysts on surface of mantle & gill tissues due to a haemocytic response; reproductive effort reduced | No curative measure. Prevention & site selection; monitoring clam rearing areas & transfer |
| Invertebrate parasites | Cercaria elegans and Cercaria tapidis | Cercarian parasite | Genital gland colonized, while a few invade the digestive organ & the surrounding areas; genital gland is affected most seriously; reproductive effort as well as growth are negatively impacted | No curative measure. Prevention & site selection; monitoring clam transfer |
| - | Proctoeces orientalis | Fellostomid trematode | Found in clam kidneys | No curative measure. Prevention & site selection; monitoring clam transfer |
Suppliers of Pathology Expertise
Assistance can be obtained from the following sources:
- European Shellfish Zoosanitary Reference Laboratory, IFREMER La Tremblade, BP 133, 17390 La Tremblade, France.
- University of Western Brittany (UBO), LEMAR Laboratory, IUEM Technopole Brest Iroise, 29280 Plouzane, France.
- Faculty of Applied Marine Science, Cheju National University, 1 Ara 1 Dong, Jeju City, Jeju Do 690-756, Republic of Korea.
- Instituto de Investigaciones Marinas Consejo Sup. de Invest. Cie., Eduardo Capelo 6, 36208 Vigo, Spain.
- Department of Fisheries & Oceans, Pacific Biological Station, Nanaimo, BC, Canada V9R 5K6
- CEFAS, Weymouth Laboratory, Barrack Road, The Nothe, Weymouth Dorset, DT4 8UB, UK.
Statistics
Production Statistics
Global Aquaculture Production of Ruditapes Philippinarum
(FAO Fishery Statistic)
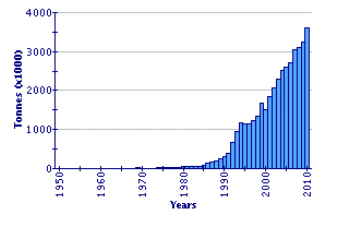
Since 1991, global Japanese carpet shell production has shown a huge expansion, by a factor of nearly six times. It now represents one of the major cultured species in the world (2.36 million tonnes in 2002). China is by far the leading producer (97.4 percent in 2002). Disease factors have impacted production in some other countries, notably in the Republic of Korea. In the decade 1993–2002, production in that country varied between 10 000 and 19 000 tonnes. Production in Italy, following its introduction, the development of wild populations, and the consequential increase in seed supply, is the second highest in the world, at over 41 000 tonnes in 2002. Other countries producing more than 1 000 tonnes in 2002 were the United States of America and France. Extensive production also occurs in Japan but is not reported within this specific statistical category, being included within 'clams, etc. nei'.
Market and Trade
In China, the Japanese carpet shell is one of the most common seafoods in coastal waters. Most clams are sold live in local markets for about USD 0.5/kg. They are either stir fried or used in soup. Clams are also marketed under different products - frozen using depurated clam and vacuum packed and frozen in a microwave oven-ready plastic bag. This species is also exported to Japan. In France and the United Kingdom, all cultured clams are sold fresh to local markets and restaurants. In Italy, clams are sold on the domestic market but large quantities are also exported to Spain. In Ireland, the low demand prompted the producers to export their fresh products to France and Spain. Since the supply has been increasing in European waters from the capture fisheries, Japanese carpet shell prices have inversely decreased from USD 10/kg to USD 8.5/kg, and later to USD 5/kg in 1983, 1987 and the 1990s, respectively.
Status and Trends
Presently, all the cultural practices related to the Japanese carpet shell (Manila clam) are under-control, including hatchery production, and the species has been widely used worldwide. The biological characteristics of the species favour further developments. Therefore, the aquaculture production of Ruditapes philippinarum is likely to increase in the near future, either through expanding acreage or by new introductions into suitable areas and countries. However, the main factor that has caused production changes in several countries has been the impact of disease. It is considered that Perkinsus –like microorganisms have been responsible for the decline in Japanese carpet shell production in the Republic of Korea, with mass mortalities occurring yearly in late summer since the early 1990s, as well as in Japan and China (Yellow Sea). Moreover, the 'brown ring' bacterial disease has slowed down production in several traditional producing countries (European Atlantic waters). Besides disease problems, the development of wild populations following the introduction of this species has induced several changes in production trends, either by facilitating the seed supply (Italy) or in contrast, by competing economically with culture (France) then favouring public fisheries. Along the western North American coast, non-indigenous predators (green crabs) pose a potential risk for commercial production.
Main Issues
The occurrence of perkinsosis in the Republic of Korea, Japan and China, may be a major threat for the culture of this species, as shown by the currently declining Korean production and abnormal mortalities in European southern waters. Future production will depend on the way zoosanitary measures are enforced to limit disease expansion. Along the western coast of North America, non-indigenous predators (green crabs) pose a potential risk for commercial production.
Considered as an exotic species and showing an expanding pattern in several countries, Japanese carpet shell can represent a threat for local biodiversity. Natural hybridization has been reported between the native European species Ruditapes decussatus and the exotic R. philippinarum. Similarly to other bivalve filtering species, the extent and scale of impact of the various biotoxins and the inability to control algal toxins is a major limiting factor for its culture. Bioaccumulated toxins can cause to long industry closures and sales prohibitions, therefore impacting the shellfish farming economy.
Ruditapes philippinarum has become a recent candidate for polyculture. Clams have been cultured with marine shrimp [Marsupenaeus (Penaeus) japonicus and Fenneropenaeus (Penaeus) penicillatus]; in fertilized seawater ponds with red tilapia (Oreochromis mossambicus*O. niloticus); combined with shrimps (M. japonicus) and European seabass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata); as well as in the drainage canals of shrimp ponds. Rotational culture of R. philippinarum has also been carried out with Porphyra culture. Polyculture has proved feasible and is a way of limiting the environmental impacts of aquaculture.
Responsible Aquaculture Practices
Epizootic events in shellfish culture have demonstrated the need for preventative measures to avoid the spread of disease, including:
- Monitoring clam populations for health.
- Establishing zoning systems to limit the spread of parasites.
- Using appropriate management practices when transferring or introducing potential species for aquaculture.
Abnormal clam mortality rates reported in Asia, and also in European waters, associated with perkinsosis demonstrate the need for rapid implementation of these preventative measures.
Aquatic Animal Health Code. Since Perkinsus atlanticus has been listed, any clam transfers should be carried out with great care. Although not listed, Vibrio tapetis is also of concern and monitoring is recommended.
Implementation of the FAO Code of Conduct for responsible fisheries (Article 9 – Aquaculture development), the ICES Code of Practices for Introduction and Transfer of Marine Organisms and recommendations for a sustainable aquaculture from the Convention of Biological Diversity are of particular importance for this species.
Japanese carpet shells have unintentionally colonized coastal areas in several countries where clam culture has been developed. Therefore, it might be considered as a pest in specific environmental conditions.
October 2012





