Small plastic debris is ubiquitous in the aquatic environment, contaminating coastal deep-sea, near-shore and open-ocean pelagic habitats. Global trends suggest that accumulations are increasing in aquatic habitats consistent with trends in plastic production— increasing 560 fold in just over 60 years6. Production trends in combination with increasing environmental accumulations may lead to greater hazards for wildlife.
Hazards associated with plastic debris include physical components of the material, chemical ingredients and sorbed environmental chemicals (e.g. persistent bioaccumulative and toxic substances (PBTs) and metals). Upon ingestion, microscopic plastic fragments can translocate into the tissues of mussels and cause increased granulocytomas and decreased lysosomal membrane stability. Based upon the UN Globally Harmonised System, >50 per cent of plastics are associated with hazardous monomers, additives and chemical byproducts (e.g. the carcinogenic polyvinyl chloride (PVC) monomer is the building block for the PVC piping that transports our drinking water). PBTs, found on recovered plastic debris globally, bioaccumulate in foodwebs and are linked with several adverse effects including endocrine disruption, decreased fish populations and reduced species evenness and richness.
A concern often raised, that remains poorly understood, is the extent that chemicals associated with plastic debris, via environmental sorption or the manufacturing process bioaccumulate in animals as a consequence of ingestion. Evidence from laboratory studies include the bioaccumulation of polybrominated diphenyls (PBDEs), a flame-retardant added to plastics, in crickets via ingestion of polyurethane foam and greater concentrations of polychlorinated biphenyls (PCBs) in lugworms fed polystyrene with sorbed PCBs. In nature, plastics with sorbed chemicals are found globally from coastal areas to the remote habitats of the subtropical gyres. Evidence from observational studies in nature have found that birds with plastic in their stomachs have greater concentrations of lower chlorinated PCBs in their tissue than those that do not and similar congener patterns of PBDEs in their tissues as those found on the ingested plastic. Of greater concern, is the hazards to wildlife health when they are exposed to the complex mixture of plastic material and plastic-associated chemicals (including the chemical ingredients and those sorbed from nature).
The physical and chemical hazards outlined above combined with the ingestion of plastic by a large range of aquatic organisms across multiple trophic levels and the evidence that supports chemical transfer from plastics to wildlife prompted us to measure the bioaccumulation of chemicals and adverse health effects from plasticingestion in fish. Fish, one of the largest and most diverse groups of animals and of great ecological- and commercial- importance, are useful as sensitive indicators of effects associated with stressors in aquatic habitats. Furthermore, plastic particles are reported in the gut content of several species of fish globally including from pelagic habitats estuaries, and bays.
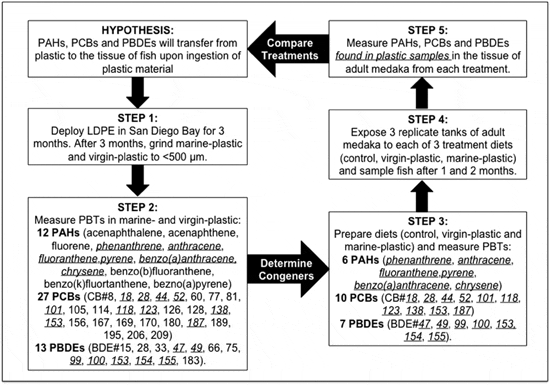
Using Japanese medaka (Oryzias latipes), a widely accepted model fish species, we achieved baseline information regarding the bioaccumulation of PBTs and associated health effects in fish via a chronic dietary exposure to low-density polyethylene (LDPE) plastic. Polyethylene has a greater affinity for organic contaminants than other mass-produced polymers, comprises the largest component of plastic production globally (29 per cent) and is one of the most common polymers recovered as aquatic debris. Fish were exposed to three treatments: a negative control (no LDPE), a virgin-plastic (LDPE virgin pre-production plastic) and a marine- plastic treatment (LDPE deployed in an urban bay). Medaka were exposed to 10 per cent plastic (by weight) mixed into treatment diets and sprinkled at the top of each tank. Diet and plastic dissociated at the surface and thus fish were exposed to plastic similar to the way they are in the wild (i.e. floating in the water column). As such, this translates to 8 ng of plastic per mL of water. Maximum concentrations reported in the North Pacific Subtropical Gyre are 300 ng/mL, and thus the concentrations of plastic used in this experiment may be considered environmentally relevant. Our chemical analyses targeted polycyclic aromatic hydrocarbons (PAHs), PCBs and PBDEs (see Figure 1 for a schematic diagram). All accumulate on plastic debris in marine habitats. In addition, PBDEs are additives on several plastics36 and PAHs are a likely byproduct of plastic manufacturing.
We hypothesized that, after a 2-month exposure, there would be larger concentrations of PBTs in the tissue of fish exposed to marineplastic and that hepatic stress would be observed, using histopathology, in fish exposed to virgin- and marine-plastic. In addition, because a healthy liver is critical for metabolizing organic contaminants, aided by the CYP1A enzyme, we hypothesized greater expression of CYP1A, using RT-qPCR, in medaka exposed to virginand marine-plastic.
Results
A cocktail of pollutants: plastic and PBTs in treatment diets. In a previous study, we found that polyethylene sorbs greater concentrations of PAHs and PCBs than other mass-produced polymers. Thus, to prepare treatments, LDPE was deployed off of a floating dock in San Diego Bay, CA for three months in preparation for the dietary exposure. 12 PAHs, 27 PCBs and 13 PBDEs were targeted in virgin- and marine- LDPE pellets and 6 PAHs, 10 PCBs and 7 PBDEs were detected and quantified (Figure 1). Concentrations of total PAHs, PCBs and PBDEs on marine-LDPE were 4 X, 15 X and 1.4 X greater than on virgin-LDPE pellets respectively (Table 1). For concentrations of individual congeners see Supplementary Table 1.
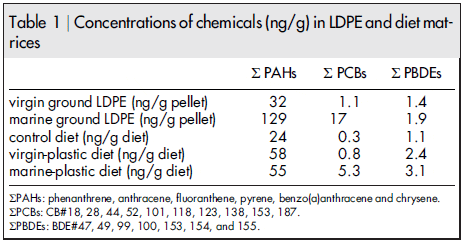
PAH, PCB and PBDE congeners quantified on LDPE were measured in all treatment diets (negative control, virgin-plastic and marine- plastic). Due to the presence of cod liver oil in all diets, PAHs, PCBs and PBDEs were present in all treatments including the negative control (Table 1). Still, the addition of LDPE deployed in San Diego Bay assured that the marine-plastic treatment diet exposed fish to greater concentrations of PBTs than the negative control and virgin-plastic treatment diets. Moreover, the addition of virgin- LDPE contributed greater concentrations of PAHs and PBDEs to the virgin-plastic than the negative control treatment. In this way, fish were exposed to greater concentrations of PBTs as a direct result of the addition of plastic to their diet. The virgin-plastic treatment diet had 2.4 X greater total PAHs, 2.2 X greater total PBDEs and relatively similar concentrations of total PCBs as the negative control treatment diet. The marine-plastic treatment diet had 2.3 X greater total PAHs, 6 X greater total PCBs and 2.8 X greater total PBDEs relative to the negative control treatment diet (Table 1). Our results show similar concentrations of PAHs reported in the marine-plastic and virgin-plastic treatment diets although concentrations of PAHs were much greater in marine- compared to virgin-LDPE pellets. Due to the complex matrix of the diet, we achieved poor resolution for several PAH congeners. In particular, fluoranthene was found at much greater concentrations on LDPE deployed in San Diego Bay, which is consistent with results from our previous study, but the poor resolution in the peaks did not allow us to detect this difference among diet samples. Individual congeners measured in all diets can be found in Supplementary Table 1.
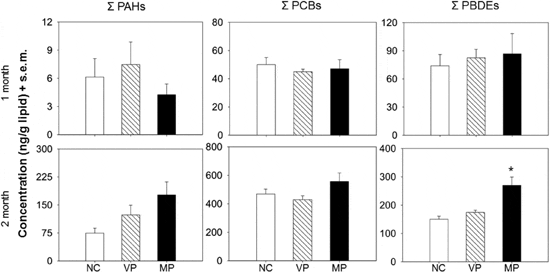
Bioaccumulation of PBTs via dietary exposure to treatment diets. After two months of dietary exposure, general patterns show a greater concentration of PBTs in fish exposed to the marine-plastic treatment. Mean concentrations of total PAHs, PCBs and PBDEs in fish from the marine-plastic treatment were 2.4 X, 1.2 X and 1.8 X greater respectively than in fish from the negative control treatment (Figure 2). While the total concentrations of PAHs and PCBs were not significantly different among treatments (P = 0.234 and P = 0.118 respectively; Figure 2; see Supplementary Table S2 for ANOVA tables), concentrations of chrysene (P = 0.006) and PCB28 (P = 0.022) were significantly greater (α = 0.05) in fish exposed to the marine-plastic treatment relative to the virgin-plastic and negative control treatments (Supplementary Tables S1 and S2). Nevertheless, total concentrations of PBDEs (P=0.0003) and all individual PBDE congeners (P < 0.05), with the exception of BDE155 (P = 0.425), in fish are significantly different among treatments such that fish exposed to the marine-plastic treatment have significantly greater (α = 0.05) concentrations of PBDEs than both the virgin-plastic and control treatments (Figure 2, Supplementary Tables S1 and S2). While we observed greater concentrations of PBTs in fish exposed to marine-plastic, this pattern was only apparent after the full 2-month exposure (Figure 2). There were not significant differences among treatments at the one-month sampling period (P > 0.05; Figure 2 and Supplementary Table S3) suggesting that short-term exposures to a 10 per cent plastic diet may not be a significant source of PBTs to aquatic life.
Adverse health effects. Hypotheses regarding adverse effects to fish focused on sublethal effects to the liver induced by the ingestion of plastic. Here, we exposed fish to a low-dose of chemicals sorbed to plastics over a relatively long time period. Thus, as expected, we did not observe large amounts or differences in mortality among treatments. Rates of mortality within each treatment, throughout the 2-month experiment, were 4 per cent from the negative control and virgin plastic treatments and 6 per cent from the marine plastic treatment.
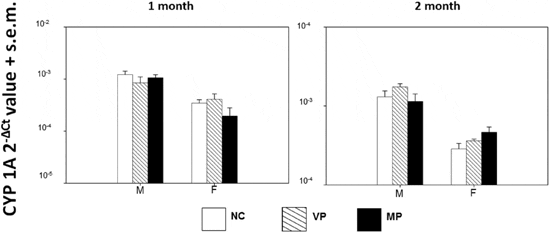
The Aryl hydrocarbon (AhR) receptor is activated upon exposure to several PBTs (including PAHs, PCBs and PBDEs) as a defense mechanism to aid metabolization39. After activation, AhR translocates to the nucleus to dimerize with ARNT, leading to changes in xenobiotic response-related gene transcription40. Transcriptional induction of CYP1A is a sensitive and specifically adaptive response in fish after exposure to polycyclic and halogenated aromatic hydrocarbons40,41 and plays a role in detoxification and/or metabolic activation (carcinogenesis) of exogenous compounds42. Because response can differ among genders when fish are reproductively active, associated with biochemical changes during spawning43, expression among treatments was compared separately for each sex. After one and two months of exposure, 1-factor ANOVAs showed no significant differences in expression of CYP1A (expressed as 22DCt) among treatments for both males (P 5 0.436 (1 month), P 5 0.324 (2 month)) and females (P 5 0.254 (1 month), P 5 0.118 (2 month)) individually (Figure 3).
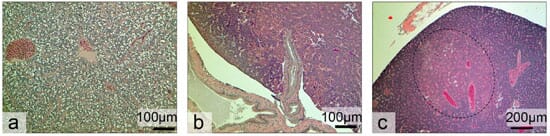
Still, fish exposed to virgin- and marine-plastic treatments show signs of stress in their livers, including glycogen depletion, fatty vacuolation and single cell necrosis. Severe glycogen depletion was seen in 74 per cent of fish from the marine-plastic treatment (n = 19 fish), 46 per cent of fish from the virgin-plastic treatment (n = 24 fish), and 0 per cent of fish from the control treatment (n = 24 fish). Fatty vacuolation was seen in 47 per cent of fish from the marine-plastic treatment, 29 per cent of fish from the virgin-plastic treatment and 21 per cent of fish from the control treatment. Single cell necrosis was seen in 11 per cent of fish from the marine-plastic treatment and in 0 per cent of fish from the control and virgin-plastic treatment. An eosinophilic focus of cellular alteration, a precursor to a tumor, was seen in one fish from the virgin-plastic treatment (Figure 4b) and a tumor, a hepatocellular adenoma (comprising 25 per cent of the liver), was seen in one fish from the marine-plastic treatment (Figure 4c).
Discussion
One question often asked is whether plastic debris is a vector for PBTs to bioaccumulate in organisms that ingest it. The objective of this work was to address this question using a medaka fish model, as several species of fish in the wild ingest plastic debris. Our model included environmentally relevant factors—LDPE fed to fish contained concentrations of PBTs that sorbed to LDPE from seawater in an urban bay, fish were chronically exposed to plastic similar to the way they are exposed in aquatic habitats, and fish were exposed to environmentally relevant concentrations of plastic. We corroborated our first hypothesis, concluding that plastic deployed in the marine environment does serve as a vector for the bioaccumulation of PBTs sorbed to plastic, suggesting that plastic debris serves as a vector for the bioaccumulation of PBTs in wildlife.
While our experiment showed a general pattern, whereby concentrations of PAHs, PCBs and PBDEs were greatest in fish exposed to the marine-plastic treatment for 2 months, concentrations among treatments were only significantly different (α = 0.05) for chrysene, PCB and all PBDE congeners except BDE155. Because PAHs are easily biodegradable, bioaccumulation concentrations do not always reflect exposure and thus the weak pattern shown here for PAHs may be expected. Due to the ubiquity of PCBs in the environment, it is difficult to differentiate bioaccumulation via plastic from bioaccumulation via the foodweb, consistent with the difficulty observed here in differentiating PCB bioaccumulation from plastic versus from cod liver oil. Still, the significant effect shown here for a lower-chlorinated PCB congener (PCB28) is consistent with observations of plastic ingestion by seabirds in the laboratory and in nature, both finding a positive relationship between the bioaccumulation of lower-chlorinated PCB congeners and plastic debris. Moreover, the strong pattern for PBDEs observed here is consistent with previous observations showing a pattern between PBDEs and plastic ingestion in wild seabirds.
One might expect to see a similar pattern for PCBs and PBDEs due to their similarities in hydrophobicity. The pattern was the same, and we observed greater concentrations of PCBs and PBDEs in fish fed the marine-plastic treatment than the virgin-plastic and negative control treatments; however, the effect size for PCBs (P = 0.1) was not large enough to be considered significant at α = 0.05. PBDEs are less stable than PCBs, breaking down into their lower brominated congeners, and biomagnifying less than PCBs. Because PBDEs do not biomagnify as well as PCBs, making exposure from prey smaller than for PCBs, contribution of PBDEs from plastic may be easier to clearly observe than the contribution of PCBs. Furthermore, the instability of PBDEs may also mean they are more likely to transfer from plastic to an organism than PCBs, whose higher-chlorinated congeners have been shown to have a strong bond to plastic debris. Testing this hypothesis may improve understanding of the patterns observed here.
There may have been greater differences among treatments if the diets formulated did not include cod liver oil and were free from contamination of PAHs, PCBs and PBDEs in the absence of plastic. Still, the situation in this laboratory experiment is ecologically relevant to wildlife because of the ubiquity and persistence of PBTs in water, sediments and foodwebs globally. Because there were not statistically significant differences among concentrations of PAHs, PCBs or PBDEs between the virgin-plastic and control treatments, we conclude that the PBTs sorbed to plastic from ambient seawater did transfer from plastic to medaka upon ingestion. Thus, results from this experiment suggest that a chronic exposure of plastic debris in nature may be a significant route of exposure for PBTs in wildlife despite globally contaminated habitats. We suggest that future work test this hypothesis.
Another frequently asked question is how plastic ingestion affects the health of fish. This experiment demonstrates hepatic stress in medaka exposed to plastic, with a greater effect in fish exposed to the combination of plastic and sorbed contaminants. Thus, future assessments regarding hazards associated with plastic in aquatic habitats should consider the complex mixture associated with aquatic plastic debris.
Fish in this experiment were exposed to a complex mixture of chemicals via plastic ingestion, including targeted PAHs, PCBs and PBDEs in addition to constituents of the plastic itself and other nontargeted chemicals (including metals) that sorb to LDPE from the marine environment. Not all chemicals activate AhR activity, and some act as inhibitors. For example, fluoranthene (detected in the diets fed to medaka in this study) inhibits AhR activity and can decrease expression of CYP1A. Increasing evidence indicates that multifactorial mechanisms might be involved when organisms are exposed to a complex mixture. Our results suggest that an enhanced and/or inhibitory metabolic compensatory response elicited during this chronic 2-month exposure, with various modes of action, may be the reason for the insignificant differences in AhR activity. For further evidence regarding effects from exposure, we examined the livers of experimental medaka for tissue damage. Because the liver plays a central role in the metabolism and detoxification of xenobiotics, liver damage may hinder induction of such mechanisms including AhR activation.
Using histology, we observed severe glycogen depletion, fatty vacuolation, cellular necrosis, and lesions. Severe glycogen depletion has been observed in fish exposed to organochlorinated xenobiotics, like PCBs, and is attributed to the direct effect of the chemical on carbohydrate metabolism and is likely linked to the energy cost of detoxification. Fatty vacuolation can lead to fatty liver degeneration and has been reported upon exposure to PCBs in rats. Although the number of lesions observed in fish is low, the formation of preneoplastic and neoplastic lesions observed in fish from the virgin- and marine-plastic treatments are likely related to the plastic. No lesions were observed in fish from the control treatment and the formation and promotion of spontaneous tumors is very rare in medaka less than one year of age.
Overall, we conclude that polyethylene ingestion is a vector for the bioaccumulation of PBTs in fish, and that toxicity resulting from plastic ingestion is a consequence of both the sorbed contaminants and plastic material. Thus, hazards related to plastic debris are not one-sided – supporting the idea that the mixture of plastic and sorbed pollutants associated with plastic debris should be acknowledged in aquatic habitats. Future studies should examine the hypothesis that plastics are a multiple stressor in aquatic habitats, shifting the focus to health effects from the combination of: the type, size and shape of the material, the chemical ingredients and the concentration of chemicals that sorbs to the material from the environment. Research that can prioritize those plastics associated with the greatest number of priority pollutants via their chemical constituents (e.g. polyurethane and polyvinylchloride) or those that sorb the largest concentrations of chemicals from the environment (e.g. PE and PP13) is suggested.
Meanwhile the waste we generate globally is accumulating faster than urbanization, 10 per cent of which is plastic, predicted to reach 2.2 billion tons annually by 2025. As such, if we continue business-asusual, by 2025 the amount of plastic discarded will surpass 220 million tons annually increasing opportunities for exposure by a wide breadth of organisms. Thus, it is time to implement more extensive research that can result in effective policy and management including the invention of materials that are sustainable and safe for people, the environment and wildlife.
January 2014



