
Unreliable maturation and spawning in captivity remain major stumbling blocks in both commercial aquaculture production and stock enhancement programmes in the US. As a result, hatchery production of many species is based on obtaining mature gametes from wild stock, and the number of fry and fingerlings produced in this way is significant.
In 2019 the US National Fish Hatchery System distributed over 240 million eggs, juveniles and adults of 112 distinct species across 46 states. Most states also have their own propagation and stocking programmes. For example, every year the Texas Parks and Wildlife Department stocks approximately 40 million fish in public waters and the Minnesota DNR’s fish hatchery system produces over 300 million walleye hatchlings, in addition to several other species.
In most of these situations, it would be virtually impossible to spawn captive broodstock by replicating the natural stimuli and circumstances that trigger spawning, so we are forced to leave Mother Nature in charge of those details until final maturation is attained. And Mother Nature knows what she’s doing. The process leading to natural, volitional spawning in most fish involves the perception and interpretation of specific environmental conditions that, in turn, trigger a complex pathway of internal physiological developments.
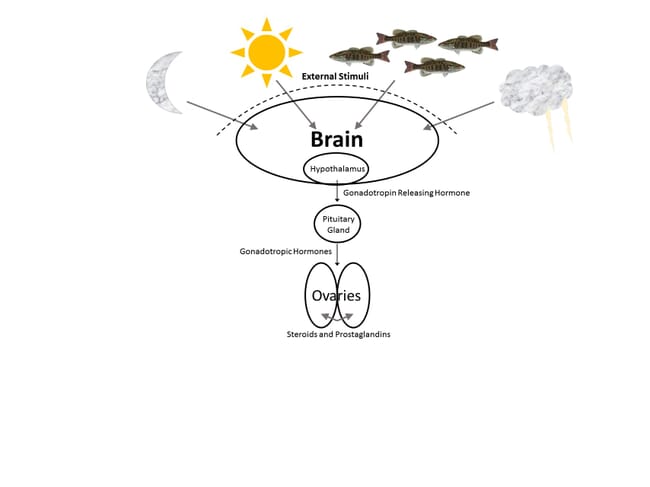
External stimuli that prompt the reproductive process in aquatic species include photoperiod, lunar cycles, ambient temperature or temperature fluctuations, precipitation events, changes in water currents and depth, changes in barometric pressure, presence and behaviours of other fish, access to suitable spawning substrates and changes in water quality – especially salinity, hardness, dissolved oxygen and pH.
Physiology and spawning
In most finfish, as external stimuli are interpreted and processed, a specific region of the brain called the hypothalamus responds by producing substances such as gonadotropin releasing hormone (GnRH) and/or substances that inhibit gonadotropin release. The brain, the hypothalamus and the compounds it produces represent the first internal links in the reproductive pathway.
The pituitary gland, typically situated directly below the brain in most fish, is stimulated by GnRH and in turn releases gonadotropic hormones (GtHs). The presence of these compounds in the bloodstream then stimulates the gonads (ovaries and testes) to produce steroids that trigger final egg maturation or spermiation, and prostaglandins that are involved in ovulation. When any link in the process is broken due to stress, injury, inadequate stimuli or other adverse effects, reproduction will not occur. If wild broodstock must be collected from natural spawning grounds, hormonal intervention may be required to prevent the disruption or complete shut-down of maturation processes as a response to capture- and transport-induced stress. One key to the success of such intervention, however, is an understanding of what links of the chain are in jeopardy and what hormonal substances can be applied to reinforce or replace the natural compounds involved.

Generally, the further along the spawning pathway artificial intervention is attempted, the greater the probability of success. This is why fish culturists the world over, who rely on wild breeding populations, must calibrate their hatchery calendars to the natural histories of the species they work with. If adult fish can be collected from their natural habitats at the proper time and location, they will have already undergone most of the physiological processes leading up to spawning. Unfortunately, even in the days or hours immediately prior to spawning, inappropriate stimuli or stressors can halt this internal chain of events. In these instances the best one can hope for is a recovery under conditions of captivity and, perhaps an opportunity for induced spawning after a subsequent maturation cycle.
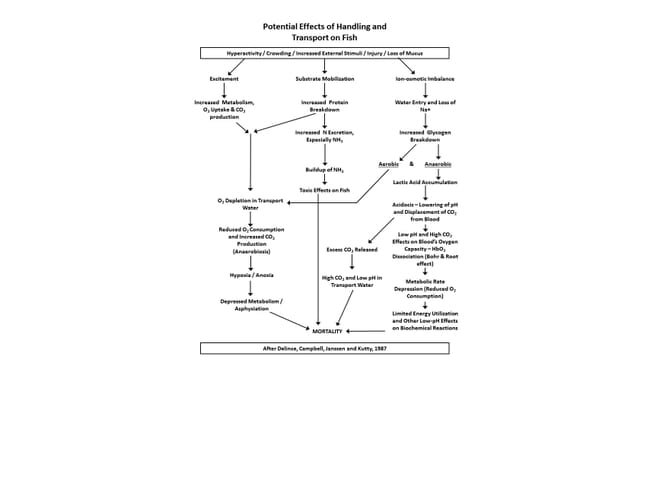
When fish are panicked respiratory rates increase dramatically, resulting in osmotic imbalance as ions are lost across gill surfaces. If fish are crowded, frightened or held under conditions that result in increased demand for and/or reduced levels of dissolved oxygen, blood may be shunted away from internal organs to circulate directly through more “vital” systems such as the heart, brain and musculature. This can result in the total loss of developing oocytes or spermatocytes, even in the advanced stages.
And, even when physical injury and oxygen deprivation can be avoided during capture or transport the release of stress-related compounds such as cortisol into the bloodstream has been implicated in the breakdown of the internal pathways that lead to final maturation, ovulation and spawning. So… hatchery personnel must not only know when and where to collect wild broodstock, but more importantly, how to collect the fish they want to propagate.
Broodstock collection methods
A number of interesting techniques have been used for a wide variety of species, and some may be applicable for many others. However, always be sure to check and comply with local and national regulations for capture and possession of breeding stock.
Collection of walleye broodstock usually begins as the water temperature rises above 4°C, generally in the early spring in many parts of North America and Europe. Trap-netting and gill-netting are the most common strategies, but success has also been reported with electro-fishing. The best approach in terms of numbers depends on the habitat the fish are being collected from, but trap-netting definitely results in significantly less stress on the fish and better spawning results. In general, larger trap nets are easier on the fish and tougher on the personnel who have to empty them.
Prepositioned electrofishing grids have been used successfully to capture robust redhorse on their natural spawning grounds. Although this technique requires visually monitoring the collection area between grids for spawning activity, it allows for egg collection and fertilisation in the field, with no need to transport adult fish to the hatchery or inject hormones prior to stripping fish. Spared the normal transport and handling stress, robust redhorse collected in this manner typically recover fully and can be released in the exact location where they are captured.
Large, strong fish - such as red drum, meagre and totaba - are easily agitated and frightened, and very prone to injure themselves during capture and transport. In the wild, red drum typically spawn every year of their adult lives, which can extend up to 40 years. Because they adapt well to captivity and photothermal conditioning, any red drum that survives capture and transport is a valuable specimen, even if stress precludes spawning immediately following collection. Active hook-and-line collection from autumn spawning aggregations has emerged as the least stressful method, if done properly. Rods, reels, lines and leaders should all be suitable for landing a 25 kg fish with minimal struggle. Barbless circle hooks help minimise injury and handling stress. As is the case with many other large species, a 20-guage (or larger) syringe may be required to deflate expanded gas bladders of fish brought to the surface from depths of 10 m or more.
General considerations
During the period leading up to spawning, physiological capacities of female fish are often pushed to the limit, as a result of the additional metabolic demand associated with ovarian development. As a result, their ability to adjust to deficiencies in water quality variables – such as ammonia, nitrites, or extremes of pH – may be severely compromised as well.
Minimise the time fish spend in any type of net, even in large trap nets, prior to transport. When collecting fish with hook and line remember that fighting a fish for a prolonged period prior to landing will leave it exhausted and in severe physiological stress. Tanks and tubs in boats and trucks must accommodate the full length of the fish being moved, and be large enough to hold not only the fish, but also sufficient volumes of water to avoid oxygen or ammonia problems.
Aeration and pure oxygen (via airstones or diffuser hoses) are essential for minimising stress on valuable broodstock. Dissolved oxygen levels in all transport containers should be maintained between 5 and 9 mg/L. Pure oxygen will be converted to highly soluble carbon dioxide, which can reach dangerous levels in transport tanks, so agitators may be needed to prevent its accumulation.
Although some antifoaming agents (silicone-based surfactants) are commercially available to reduce foam buildup in fish hauling tanks, the presence of excess foam on the water surface is usually an indication of excessive fish loads and mucous loss resulting from handling stress. If suitable water quality cannot be maintained during transport, plans should be in place to allow for partial water exchanges en route.
Temperature will influence the number of fish that can be transported, as will the total travel time in the boat and over the road. Reducing the temperature by several degrees usually reduces losses during and after transport. Most freshwater fish will benefit from the addition of 0.5 percent salt (sea salt or un-iodized food grade salt) to the transport water. Saltwater fish will usually benefit from a reduction of 2 or 3 ppt in salinity. However, to reduce time spent in transport tanks, it is critical to avoid unnecessary acclimation procedures. Make every effort to minimise differences in water chemistry between the collection site, the transport tanks and the receiving facility.

© LA Fisheries Forward
Stress reduction
To the extent possible, bright light should be avoided, so shade cloths or more solid covers should be used on collection and hauling tanks. Regulations will vary from state to state and country to country, but anesthetics are often used to lightly sedate fish during transport to reduce stress and injury. Buffered tricaine methanesulfonate (MS-222), purified clove oil or metomidate hydrochloride can be added to transport tank water to calm captured broodfish.
Round transport tanks are generally more suitable than square or rectangular ones, especially for larger species, but they are rarely practical due to space considerations. Holding tanks can be lined with soft materials to reduce self-inflicted damage during transport, but potentially toxic materials such as upholstery foam should be avoided. A variety of fish-safe materials such as foam filter mats can be temporarily or permanently fitted to interior tank walls.
Knotless netting or rubberised dipnets should always be used to minimise loss of scales and protective mucous. Similarly, hands, towels and other objects coming into contact with broodfish when they are removed from the water must be wet at all times. Large fish can be moved for short distances using wet stretchers constructed from sturdy plastic tarp material sewn onto wooden, plastic or metal poles. Covering a fish’s eyes with a wet cloth during handling will help reduce physical resistance and keep it calm.
Pathogen control
Stress from capture and handling makes fish particularly vulnerable to bacteria and any opportunistic pathogens that may be present. Antibiotics, fungicides, parasite treatments and antiseptic compounds may be required to improve the welfare and survival of captive broodstock. Always follow applicable guidelines and regulations for the region and country you are operating in, and consult a veterinarian if you are uncertain regarding dosages and application rates.








