Habitat and biology
Seabass spawn small (1.02-1.39 mm) pelagic eggs in water with salinities lower than 35‰, near to river mouths and estuaries or in littoral areas where the salinity is high (≥30‰).

Being not particularly sensitive to low temperature some fish may over-winter in coastal lagoons instead of returning to the open sea. Seabass are predators and their feeding range includes small fish, prawns, crabs and cuttlefish.
Production
Production cycle
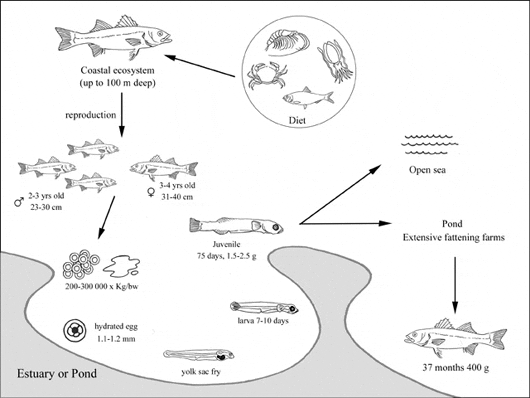
Extensive system
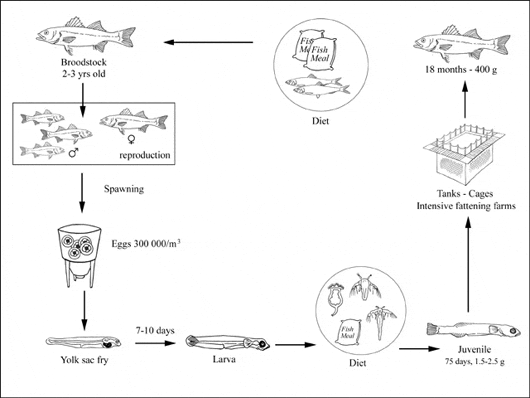
Production systems
Although seabass are farmed in seawater ponds and lagoons, the bulk of production comes from sea cage farming.
Extensive lagoon systems
The traditional extensive method of lagoon management places special barriers in appropriate lagoon sites to capture fish during their autumn migration to the open sea. Barriers made of reeds, nets or cement stay open from February until May for the lagoon to be naturally stocked with fry. In this system seabass is usually cultured in polyculture with seabream, mullets and eels. Seabass reach a commercial size of 400-500 g in 37 months, with a total lagoon production of 50-150 kg/ha/yr. The limiting factor is in the natural feeding behaviour of the seabass that, as predators, may drastically reduce the natural resources of the lagoon ecosystem.
Semi-intensive lagoon systems
These techniques involve artificial enrichment with fry, fertilization of the lagoons, and improvement projects. Specialist fishermen collect fry from coastal waters during May and June; then fry are transported in oxygenated tanks for a first stage of growing in special ponds, until they reach a size which enables them to survive in the lagoon. Projects for improved productivity involve the development of sufficient canals and making new openings to the open sea for water exchange and enrichment with plankton and fry. Peripheral ditches (with fresh or seawater) are dug for salinity control, and also wintering ditches at least 2 m deep in several areas of the lagoon. Finally, vegetation control is important in order to avoid suffocation of the fish. Losses in fish production in the lagoons are due to insufficient enrichment with fry, predation, decreased freshwater supply (due to lack of rain), and lack of sufficient improvement projects. The production is higher than in the extensive system and amounts to 500-700 kg/ha/yr.
Hatchery production
Broodstock
To secure a reliable and sufficient supply of good quality fish eggs, most hatcheries have established their own broodstock units, where breeders of different age groups are maintained long-term. Parent may come either from a farm or from the wild. The optimal age for female parent fish is between 5 and 8 years, whereas for males this range is lowered to 2-4 years. The management of captive broodstock in the breeding stations includes natural maturation, the induction of ovulation by photoperiod manipulation or hormonal treatments, fertilisation in spawning tanks and incubation in an open-water circulation system.
Spawning
At the onset of the spawning season it is necessary to move selected batches of breeders from their long term holding facilities to the spawning tanks, where they can be better treated and their performance can be easily monitored. The male:female ratio in the spawning tanks is kept at 2:1. Whereas males are chosen when they release sperm spontaneously or on stripping, the female maturation stage has to be ascertained by extracting oocytes from the ovary with the use of a catheter: only females with oocytes in the late-vitellogenic stage, i.e. with a diameter larger than 650 µm are selected.
Photoperiod manipulation
When fertilised eggs are required outside the natural spawning period, out-of-season sexual maturation is obtained by promoting gametogenesis by manipulating the photoperiod and temperature. The hatchery management decides on the periods of egg production according to its marketing and/or farm needs.
Hormonal treatment
Hormonal treatment is used to trigger the last phase of egg maturation. The human chorionic gonadotropin (HCG) is used at a dosage of 800-1000 IU per kg/bw, delivered in two injections in the dorsal muscles, 6 hours apart.
Ongrowing techniques
In intensive production, ongrowing units are supplied with fry from hatcheries and controlled diet is provided.
Juveniles are sold to farmers as ongrowing stock at a size of 1.5-2.5 g. The ongrowing juveniles reach 400-450 g in 18-24 months. Feeds are distributed by automatic feeders every 10-15 minutes for small fish (2-15 g), or by hand for larger fish. Grading is necessary at least two or three times per cycle, in order to avoid growth differentiation and cannibalism. Fattening can occur in tanks or in cages system.
Cage systems
Net-pens (cages) can be of different kinds but the principle is the same; all types are based on a natural exchange of water through pens. The quality of sites is therefore highly variable, according to local conditions such as tide and current. The cages are usually made of steel with areas of 4 to over 10 m², having nets suspended below the walkways up to 6-8 m deep. Some farms are anchored close to the land and can be served from a landing. Others are located in the open sea or in the middle of a protected bay and can only be served by boat. An important factor is the husbandry of the pens; frequent net changing is essential, especially in hot periods (every 15-20 days); weekly cleaning to remove fouling organisms and periodical treatment with anti-fouling products is also necessary. The removal of dead and moribund fish by divers is done, usually weekly but preferably daily during outbreaks of problems.
Tank systems
Tanks are usually supplied with seawater (38‰) maintained in a continuous flow-through system under ambient temperature. Alternatively, brackishwater (30‰) pumped from adjacent lagoons may be used. High stocking densities are applied (20-35 kg/m³); this means that accurate control of water quality and careful observations of fish health are essential. A recirculation system, to control water temperature (between 13-18°C) is used during autumn/winter, frequently full-time in hatchery and the pre-fattening phase of the production cycle; this system is also used for fattening in high technology farms. This practice improves growth but can be highly expensive due to the required technology for water quality control (filtering, air stripping, UV treatment, catabolite removal).
Harvesting techniques
Commonly fish are fasted for several days before slaughter, ranging between 1 to 12 days depending on seasonal variation of the water temperature. In commercial farms, either land-based tanks or cages seabass are harvested with dipnets or vacuum pumps in very high densities (70-100 kg/m³), just before killing by asphyxiation in chilled water. In the case of sea cages, harvesting is practicable only when weather conditions are acceptable for worker safety.
The time between the harvesting and slaughtering depends on the distance of the cages to the farm, usually not more than two hours, including transport. When the farm has its own slaughtering facility, fish are placed in chilled water with slurry ice and immediately slaughtered.
Prolonged crowding before harvesting is avoided, to ensure high product quality and fish welfare. Greater muscle activity at slaughter leads to a rapid decrease in energy reserves (i.e. adenosine triphosphate, ATP), and to the build up of lactic acid and consequently a drop in post-mortem pH. An animal that struggles at slaughter goes into rigor very rapidly, adversely affecting the quality of fish fillets by softening the muscle texture.
Killing methods should result in rapid and irreversible loss of consciousness. Methods that kill fish rapidly result in a reduction of stress, thus an improvement in welfare and in quality. It has been demonstrated that methods that leave the fish insensible are spiking of the brain, a blow to the head or the destruction of the spinal chord. However, these killing procedures are less practicable in commercial situations for seabass, due to the commercial size of fish and the high cost of qualified personnel.
Handling and processing
Harvesting methods vary according to the scale of the operation. The need to harvest large volumes quickly requires automation, although smaller operations can rely on manual methods. In either case the aim must be to maintain the quality of final product through careful handling, practiced by qualified personnel. Handling includes transfers from one rearing unit to another, as well as during the harvesting and transport of live fish. Hygiene should follow the general principles used in processing. Nets and tanks must be cleaned regularly. During harvesting, attention must be paid to prevent bird predation, as well as the physical damage caused by netting and pumping. The epidermis and scales are easily removed during these procedures. Care is also needed during fish packaging to prevent scale loss and to preserve the appearance and brightness of the skin. Whole fish are usually sold fresh in batches. Only a small proportion is sold frozen and packaged individually. Fresh fish are not usually kept on ice for more than 4-5 days before reaching the market.
Production costs
Fry usually comprise between 15-25 percent of ongrowing costs. In hatcheries, labour costs are ~30 percent of the total. Another large contributor is feed (30 percent), followed by administrative expenses, and fuel and power (due to the requirements for heating and automatic feeders. Generally ongrowing costs are lower in larger scale farms.
In Italy, for example, juvenile production costs are about EUR 0.30/kg (USD 0.39/kg ), and fish production, including all the above expenses, costs about EUR 4.00/kg (USD 5.20/kg).
Diseases and control measures
Although a sturdy species, seabass are subject to a wide range of diseases under rearing conditions. These outbreaks have important effects on commercial production and could prevent the expansion of the industry in some countries.
Stress is considered an important factor co-responsible for disease outbreaks; thus improved husbandry is generally suggested to reduce stress. Another problem is the lack of authorized effective therapeutants, particularly for parasites, in most European countries.
Commonly found problems are shown in the following table.
In some cases antibiotics and other pharmaceuticals have been used in treatment but their inclusion in this table does not imply an FAO recommendation.
| DISEASE | AGENT | TYPE | SYNDROME | MEASURES |
|---|---|---|---|---|
| Viral encephalo-retinopathy | Nodavirus | Virus | Nervous symptoms | Good prophylaxis; good husbandry conditions |
| Vibriosis | Vibrio anguillarum; Vibrio ordali; Vibrio spp | Bacteria | Anorexia; darkening; skin ulcers; abdominal distension; splenomegaly; visceral petechiation; necrotic enteritis | Fry vaccination; antibiotic treatment |
| Photobacteriosis or Pseudotuberculosis | Photobacterium damsela subsp. pasteurella | Bacterium | Anorexia; darkening; splenomegaly; miliary lesions of spleen or spleen granulomatosis (chronic form) | Antibiotic treatment |
| Myxobacteriosis | Flexibacter maritimus | Bacterium | Skin ulcers; necrosis; fin erosion | Antibiotic treatment |
| Mycobacteriosis | Mycobacterium marinum | Bacterium | Emaciation; poor growth; hypertrophic kidney and spleen with granulomas | Good prophylaxis |
| Epitheliocystis | Chlamydia-like | Bacterium | Miliary nodules on skin or gills | Good prophylaxis |
| Amyloodiniasis | Amyloodinium occelatum | Dinoflagellate | Skin darkening; skin dusty appearance (velvet disease) | Freshwater treatment |
| Cryptocaryoniasis | Cryptocaryon irritans | Ciliates | Skin lesions; white spot or multifocal white patches (marine white spot disease) | Freshwater treatment |
| Scuticociliatosis; other ciliatosis | Philasterides dicentrarchi; Uronema sp.; Tetrahynema sp. | Ciliates | Skin and gill lesions; depigmentation; ulcerations; skin area haemorrhages | Freshwater treatment |
| Myxosporidiosis | Shaerospora dicentrarchi; S. testicularis; Ceratomyxa labraci | Myxosporidia | Reduced production; reduced growth rate; low mortality | No treatment |
| Microsporidiosis | Glugea sp. | Microsporidia | Reduced production; low mortality | No treatment |
| Gill fluke infections | Diplectanum aequans; D. laubieri | Monogenean trematode | Skin cloudiness; focal reddening with excess mucus production; epithelial hyperplasia; gill haemorrhages | Correct prophylaxis; good husbandry condition |
| Anisakis infection | Anisakis spp. | Nematoda | Larvae in coelomatic cavity | Correct prophylaxis |
| Isopodiasis | Ceratothoa oestroides; Nerocilla orbiguyi; Anilocra physoides | Crustacea (isopods) | Growth retardation; gills and skin tissue necrosis; adults and larvae on fish | Correct prophylaxis |
March 2009




