The pH and mineral content of water are the result of interactions between the soil beneath a pond and the water used to fill it. Clay soils are often acidic. Because ponds are commonly constructed on these soils, especially in the southern and southeastern US, the effect on water quality can be significant.
Ponds with acidic bottom soils that are filled with poorly mineralised water characteristically have low alkalinity and hardness. When total alkalinity and hardness are below 20 mg/L (as CaCO3) pH and productivity are usually reduced. Alkalinity concentrations below 20 mg/L often lead to large swings in daily pH values, which stress aquatic animals. Acidic soils contain high concentrations of hydrogen ions and/or aluminium relative to the concentrations of calcium and magnesium, which are important minerals for good water quality.
The acidity of pond soils can be neutralised and the productivity of the pond improved by liming. “Liming” refers to the application of various acid-neutralising compounds of calcium or calcium and magnesium.
Liming ponds has three important benefits:
- Liming may enhance the effect of fertilisation.
- Liming helps prevent wide swings in pH.
- Liming also adds calcium and magnesium, which are important in animal physiology.
The difference between alkalinity and hardness
It is important to understand the difference between alkalinity and hardness. These two aspects of water chemistry are often confused. The misunderstanding relates to the term used to report them—ppm as CaCO3 (mg/L).
Total alkalinity indicates the entire quantity of titratable bases present in water, primarily bicarbonates, carbonates and hydroxides. The most important components of alkalinity are bicarbonates and carbonates.
Hardness is the overall concentration of divalent salts (calcium, magnesium, iron, etc) but does not identify which of these elements is/are the source of hardness. Calcium and magnesium are the most common sources of water hardness. Liming increases both alkalinity and hardness.
The effect of liming on fertilisation
Both recreational and commercial ponds are often fertilised to improve fish production. Fertilisers containing nitrogen, phosphorus and potassium (especially phosphorus) stimulate the growth of microscopic plants (phytoplankton) and animals (zooplankton), which, in turn, serve as food for animals in the aquatic food chain.
In recreational ponds, an abundance of plankton supports larger populations of species such as largemouth bass and bluegill. In ponds used for commercial production of juvenile fish, plankton is the primary food source. Healthy phytoplankton blooms also absorb toxic nitrogen wastes and raise daytime dissolved oxygen concentrations, so they are important to water quality.
Perhaps the most common reason to lime ponds is to improve the response to fertilisation. In ponds built on acidic soils and filled with fresh water of low mineral content, much of the phosphorus added in fertilisers becomes tightly bound in pond sediment where it is not available to support phytoplankton growth. Proper liming can improve phosphorus availability and greatly enhance pond productivity.
Liming and pH swings
In ponds with acidic soils, filled with poorly mineralised water with low total alkalinity, liming will increase total alkalinity. This helps stabilise pH, which can swing widely from 6 to 10 during the day if total alkalinity is below 20 mg/L.
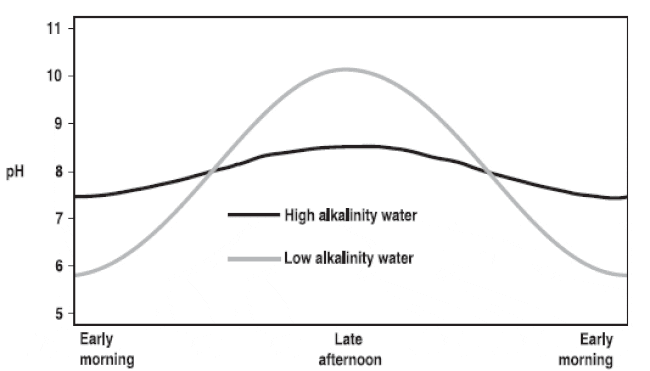
Fluctuations in pH are the result of the interplay of photosynthesis and respiration. Nighttime respiration increases CO2 concentrations, creating carbonic acid and causing pH to fall. During the day phytoplankton absorbs CO2 for photosynthesis, causing pH to rise. Large, daily changes in pH can stress aquatic animals (Figure 1).
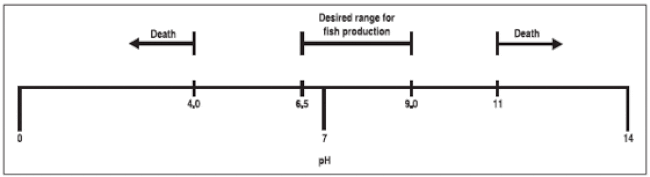
Most aquaculture species can live in a broad range of alkalinity concentrations, but the desired alkalinity for many animals is 50 mg/L or higher. Liming to raise total alkalinity to the required or preferred ranges buffers the water and reduces swings in pH (Figure 2).
Liming and hardness
Hardness concentrations are important to aquatic animals also. Calcium and magnesium are essential for bone and scale formation in fish. The most critical component of total hardness, however, is the calcium concentration or “calcium hardness.”
Environmental calcium is crucial for osmoregulation, the biological process that maintains precise levels of internal salts for normal heart, nerve and muscle function. In low-calcium environments, animals can lose (leak) substantial quantities of these salts into the water. Calcium is also important in the moulting process of shrimp and other crustaceans, and can affect the hardening of newly formed shells.
Most aquatic organisms can tolerate a broad range of calcium hardness concentrations, but a desirable range is 75 to 250 mg/L with a minimum concentration of 20 mg/L. Adding liming materials or gypsum increases hardness.
© Wurts and Durborow, 1992
Deciding whether to lime a pond
To determine whether a pond needs to be limed, first check total alkalinity. Collect a water sample from the first several inches below the surface, making sure the sample contains no bottom sediment (mud). Collect the sample in a clean quart container that has no chemical residues. The sample can be tested for total alkalinity with a swimming pool test kit. Or, the sample can be sent to a university laboratory or commercial testing company. If you are in the United States, check with your county Extension agent for information about water testing.
If the total alkalinity of the water sample is less than 20 mg/L, the pond may benefit from liming. The amount of lime needed depends on the chemical characteristics of the bottom sediment. Take samples of the pond bottom and have them analysed to determine the soil pH and the amount of liming material to apply.
Collect the samples as you would for cropland. Take samples to a soil depth of 6 inches from several locations in the pond (an S-shaped pattern is usually used). In ponds less than 5 acres, collect at least ten samples per acre. In larger ponds, collect four to eight samples per acre.
In a new pond, collect soil samples before filling. In ponds with water, push a length of PVC pipe into the bottom and remove the mud plugs from the pipe. Or, attach a can or small container to a long pole and scoop soil from the pond bottom. Combine the samples, mix them evenly, and spread the blended sample out to dry. After drying and crushing, mark the sample “pond mud” so the appropriate analysis can be made.
Approximately 1 pint of dried, blended soil sample is needed for lab analysis. Contact your county Extension agent for information about soil testing services.
In some areas, specific tests for “pond mud” are not available. However, there is a simple and reasonably accurate way to estimate the amount of liming material needed in a pond. Submit the sample and request the recommendation for alfalfa production. The amount of liming material needed to grow alfalfa will be very close to the minimum required for producing most aquatic animals. Another method is to apply 11/2 to 2 times the amount of liming material used to farm row crops in the surrounding area.
© Wurts and Durborow, 1992
Choosing liming materials
Materials such as agricultural limestone, basic slag, slaked lime, quick lime and liquid lime have been used to lime ponds. While all these compounds neutralise soil acidity, some are more practical or effective than others.
It is not advisable to use quick lime (CaO) or slaked lime (Ca(OH)2). They are more expensive and can cause pH to rise rapidly to levels that can harm aquatic life.
Basic slag is a satisfactory liming material, but it is not commonly available and its effectiveness may vary significantly from load to load. A substance known as silicate slag is not an acceptable material and should not be used to lime recreational or commercial production ponds.
Liquid lime is popular among some farmers. This product is made by suspending finely powdered agricultural limestone in water. The small particles react more rapidly with the acid in soil and water and produce quick results. However, because this mixture is half water, it takes twice as much liquid lime as agricultural limestone to achieve the same results. Liquid lime can cost much more than agricultural limestone.
Finely crushed agricultural limestone is usually the best material to use. It is cost-effective and readily available. Both pond alkalinity and hardness can be increased by adding either CaCO3 (calcitic) or CaMg(CO3)2 (dolomitic) limestone. It is difficult to add too much agricultural limestone to a pond. At a pH of 8.3 or greater, calcium combines with carbonate to form limestone and drops out of solution. Limestone does not dissolve well in ponds where soil acidity has been neutralised and water pH has stabilised at or above 8.3.
Neutralising value and efficiency
Commercial liming materials vary in their ability to neutralise soil acidity—their neutralising value (NV). Pure calcium carbonate is the standard used for assigning relative neutralising values to each of the liming compounds.
Calcium carbonate is considered to have an acid neutralising value of 100 percent. Agricultural limestone may have NV values between 85 and 109 percent depending on its specific chemical composition. Slaked lime has an NV of 136 percent. Neutralising values for the liming materials previously discussed may fall between 55 and 179 percent (Table 1).
Finely crushed agricultural limestone is composed of different sizes of particles. Small particles react faster and dissolve more rapidly and completely than large particles. Therefore, the neutralising efficiency (NE) of agricultural limestone depends on the fineness of the mixture.
The particle fineness and associated neutralising efficiency are determined by passing limestone through a series of sieves. Particles that pass through a 20-mesh sieve but that are retained by a 60-mesh sieve have an NE of 52.2 percent. Those passing through a 60-mesh sieve have an NE of 100 percent. The various quantities of each particle size grouping and their associated NE values must be averaged to arrive at an overall NE rating.
If the liming requirement, neutralising value (NV) and neutralising efficiency (NE) are known, it is possible to calculate the precise amount of lime needed. Divide the amount of liming material recommended (tonnes per acre) by the product of the neutralising value and the neutralising efficiency (NV x NE).
For example, a farmer submits a soil sample and the analysis indicates the 3 tonnes per acre of pure calcium carbonate are required to neutralise the pond soil acidity (or to produce alfalfa). The agricultural limestone available at the local farm supply store has an NV of 85 percent and an NE of 71 percent. The amount needed is determined as follows (percent must be converted to a decimal first):
tonnes/acre CaCO3 divided by (NV multiplied by NE) = tons of limestone needed
3.0 ÷ (0.85 x 0.71) = 4.97 tonnes of limestone needed
When only one value is available (NV or NE), divide the tonnes recommended by that value. For example, if only the NV (85 percent) is known:
3.0 ÷ 0.85 = 3.53 tonnes of limestone needed

*Use of these materials is not recommended because their effects on pH can be harmful to aquatic life.
Timing and application of liming materials
To be effective, liming materials should be applied evenly over the bottom of the pond. The best, and easiest, time to lime a pond is before it is filled with water. A liming truck or tractor-pulled liming wagon can be driven around in the dry pond to spread the lime evenly over the entire bottom. It is not necessary to disc the lime into the soil, but this will accelerate its neutralising activity.
If the pond contains water, lime should be applied evenly over the entire pond surface. Lime is loaded onto a boat or barge and then shovelled or washed uniformly into the pond (Figure 3). Often a sheet of plywood can be attached across the front of one or two small boats and the lime placed on the plywood.
Lime is heavy and shovelling it is tedious. Therefore, some pond owners hire professional companies with liming barges to spread the lime. For small ponds of less than 1 acre, liming trucks can be backed up to the edge of the pond and the lime distributed with the spreader on the truck. This method works best if the truck can move around the entire pond and broadcast the lime evenly.
Agricultural lime does not dissolve quickly in water and will sink to the bottom. Liming a pond filled with water has an immediate effect on water quality. It increases pH, reduces soluble phosphorus, and reduces free carbon dioxide. Increasing the pH may cause the water to clear of suspended particles (mud), which can help pond productivity by increasing the light available to plants.
However, liming a pond shortly after fertilising may remove phosphorus from the water, which could prevent a phytoplankton bloom from developing. Recreational ponds are typically fertilised in the spring with compounds containing phosphorus. So it is usually best to apply lime in fall or winter when productivity is unlikely to be affected. The pond will equilibrate within several weeks and then fertiliser can be applied to adjust productivity.
Limestone dissolves slowly over time. Alkalinity and hardness are washed out of the pond with overflow and drainage water. Ponds that require lime usually need repeat treatments every 3 to 5 years. Alternatively, annual lime applications can be made using one-fourth the original recommendation to maintain alkalinity, hardness and pH at acceptable levels. If a pond needs lime, it will not respond well to fertiliser.

Managing calcium hardness
If the alkalinity concentration is below 50 mg/L, agricultural limestone can be used to increase alkalinity and hardness. If total alkalinity is above 50 mg/L, adding agricultural limestone will not be effective. Similarly, if pond pH is stable at 8.3 or greater, limestone will not dissolve. For several aquaculture species (eg, striped bass, red drum and crawfish), the preferred concentration of calcium hardness is above 50 mg/L.
Liming with agricultural limestone, using recommendations based on soil analysis, will usually increase alkalinity and hardness to the minimum required concentration of 20 mg/L. A low total hardness value is a reliable indication that the calcium concentration is low. However, a high hardness value does not necessarily mean that the calcium concentration is high.
Where hardness is caused by CaMg(CO3)2 (dolomitic limestone), the total hardness value reflects a mixture of calcium and magnesium. Magnesium can represent as much as 50 percent of the hardness produced by CaMg(CO3)2. Other magnesium-containing compounds, such as magnesium sulfate, may be the source of hardness in high alkalinity environments.
Therefore, agricultural limestone may not always raise calcium to the required or minimum desired concentrations. Agricultural gypsum (calcium sulfate) or food grade calcium chloride may be needed to raise calcium hardness in waters with alkalinities greater than 50 mg/L and low hardness. Where alkalinity is high and hardness is caused by magnesium, adding agricultural gypsum or calcium chloride is also an effective way to raise the calcium concentration.
Alternative materials for raising calcium hardness
Agricultural gypsum (calcium sulfate)
Calcium hardness and total hardness can be increased about 1 mg/L by applying 5 pounds of agricultural gypsum per acre-foot. Adding 125 pounds of agricultural gypsum per acre-foot would raise hardness approximately 25 ppm.
Calcium chloride
Calcium hardness and total hardness can be increased about 1 mg/L by applying 4 pounds of calcium chloride per acre-foot. Adding 100 pounds of calcium chloride per acre-foot would raise hardness roughly 25 ppm.
It is important to note that if phosphorus is added to ponds immediately before or shortly after applying gypsum or calcium chloride, the phosphorus may combine with calcium. This may cause both elements to drop out of solution as calcium phosphate. Phosphorus-based fertilisers should not be added for several weeks before or after the application of compounds that increase calcium hardness.
If high volumes of water regularly flush through a pond, the agricultural limestone, agricultural gypsum or calcium chloride that have been added can be washed out. Often more than the recommended amount of limestone or gypsum is added so the materials will not have to be applied as often. These chemicals will not cause problems in a pond if added at two or three times the calculated amount.
Fish farmers often overlook the importance of hardness and alkalinity. The pond environment and aquatic animals benefit from water that has the desired levels of alkalinity and hardness. The minimum concentration for both is 20 mg/L.
Managing these two components of pond water stabilises or buffers pH fluctuations, improves the availability of phosphorus for phytoplankton, increases the natural food in ponds, and provides calcium for osmoregulation, egg hardening and other metabolic needs.
Water should be tested periodically so that hardness and alkalinity can be managed properly. Apply liming materials as needed and keep good records to improve water quality and overall pond productivity.


