This has led to changes in agricultural and aquaculture practices that could soon take place in other regions around the world. This article reports on a field trial using Orego-Stim® Aquatract as a phytobiotic feed additive in Whiteleg Shrimp Culture P. vannamei in Panama.
In the last decade, P. vannamei has emerged to become the number one shrimp of choice for cultivation around the world. Today P. vannamei represents approximately three quarters of the world’s farmed shrimp and has replaced the previous favourite P. monodon due to a tolerance of higher stocking densities per hectare and better feed conversion ratios both of which result in higher return on investment for farmers and consequently the retail value chain.
Bio-secure shrimp production
Shrimp aquaculture continues to find ways of applying revolutionary innovation and technology to its commercial farming practices. One such revolution has been the development of specific pathogen free (SPF) shrimp. Such shrimp are designated free from one or more of a particular named pathogen which is usually one of the nine viruses’ that are can infect shrimp. SPF condition is lost once the shrimp leaves the SPF facility, unless they are transferred directly to an equal status bio-secure facility. Consequently SPF status is a temporary condition that is not passed on genetically. Specific Pathogen Resistant (SPR) shrimp is another innovative technology that provides resistance to a particular strain of virus for example.
However as is known with other mammalian and human viruses’, they have the ability to mutate into different strains and therefore SPR shrimp may not necessarily convey the same level of protection against all strains of viruses’. Similar to other organisms, there appears to be a trade-off between disease resistance and shrimp growth (Chevassus and Dorson 1990, Henryon et al. 2002, Ceniacua & Akvaforsk 2002). There are compelling reasons why breeding shrimp for resistance to a single viral pathogen, using current selective breeding strategies, may not be the most prudent course of action for the long-term viability of the shrimp farming industry (Moss et al. 2005).
Larvae nutrition
Since marine fish and shrimp farming first became commercially viable on a significant scale, the larval and hatchery phases of growth have been particularly susceptible to high levels of mortality. There are several reasons for this. It is vital to use a nutritionally suitable feed of a correct size, colour and texture that moves in such a way to attract the fish or shrimp’s instinctive feeding behaviour. Despite the development of advanced commercial pellet diets, it has been impossible to replace live feeds in the early part of the life-cycles of fish and shrimp.
Brine shrimp (Artemia spp.) are fed to shrimp larvae between the 7th and 26th days of their lives. This is a critical period to ensure high rates of survival when the fast-growing shrimp larvae need an energy-boost not available from other commercial feeds. It has been estimated that a single shrimp will consume 1,750 brine shrimp during this 2 1/2 week period. The rapid development of shrimp farming in Southeast Asia and Latin America in the 1980s and 1990s created a huge demand for brine shrimp and transformed the industry, over 80 per cent of which come from the great salt lakes of Utah, USA.
Grow-out nutrition
Once the larvae are transferred into the grow-out ponds, their challenges change from dietary nutritional composition to disease resistance in an aquatic environment that doesn’t provide the same level of bio-security as the nursery. Ballast water exchange, for example in the Panama Canal, is a significant pathway for shrimp virus transmission from wild stock. It is more difficult to assess the health of shrimp populations in the ponds than the nursery, and any delay in the lag phase in recognition of potential indications of disease can have significant unwanted consequences for the farmer in terms of mortality and lowered FCR.
Historically, one way that shrimp farms have combated disease has been with the use of antibiotics, however in recent years the EU which is a major buyer of shrimp products on the world market has changed its policy on import legislation and border inspections. Shrimp food products have consistently made up about half of the failed EU rapid alert tests in the last few years. The marker residue for the banned nitrofuran veterinary antibiotic nitrofurazone called semicarbazide has been frequently detected in foods (47 per cent of recent nitrofuran EU Rapid Alerts involve semicabazide). Importers of shrimp have reported in the past rampant use of antibiotics such as chloramphenicol and nitrofuran in aquaculture farms.
Traces of antibiotic residues in aquaculture shrimps exported in the past have prompted some of the European countries to reject several export consignments. The development and spread of antimicrobial resistant human pathogens (motile Aeromonas spp., E. tarda, Escherichia coli, V. vulnificus, V. parahaemolyticus, V. cholerae etc.) have been well-documented (WHO, 1999).
Feed treatment methods
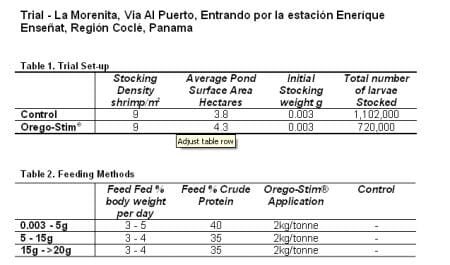
A commercial shrimp feed was used for the entire duration of the trial manufactured by a leading Shrimp feed manufacturer. Shrimp were fed twice a day at a rate of 60 per cent their daily ration in the morning and 40 per cent in the afternoon. The trial lasted for a period of 4 and a half months with post larvae stocked into five ponds, three control and two Orego-Stim® Aquatract trials. Shrimp were harvested at an average weight of between 23 and 26g. Orego-Stim® Aquatract was applied topically to the pellets prior to feeding at a concentration of 2kg per tonne dissolved into a small quantity of blended marine fish oil.
Feed conversion ration and survival rates
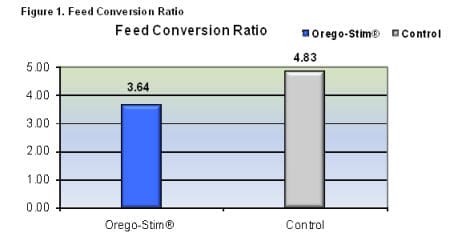
The trial was compromised by an invasion of a species of marine ghost shrimp which competed for commercial feed and had cannibalistic effects. Consequently FCR was higher than expected and survivability was lower than expected. The feed conversion ratio and survival rates are shown in figures 1-2.
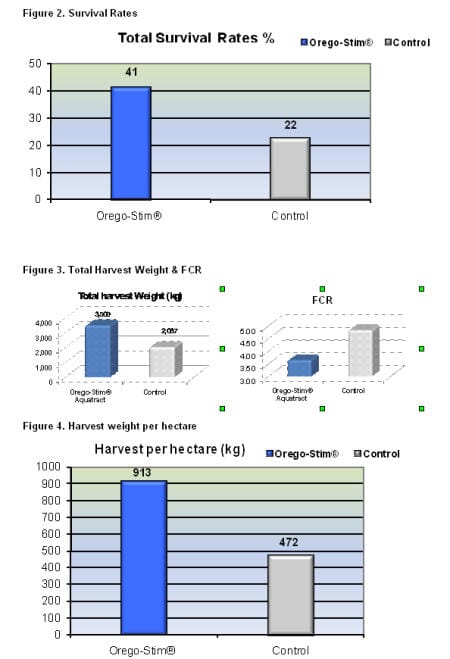
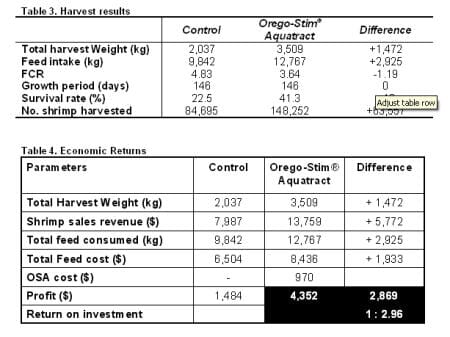
Feed conversion ratio was reduced by an average of 25 per cent in the Orego-Stim® Aquatract ponds. Total survival rates of the Orego-Stim® Aquatract ponds were almost twice the control ponds. Below, table 3 shows the huge differences in harvest parameters made between the two treatments with a 72 per cent increase in final total harvest weight and a 75 per cent increase in the numbers of shrimp harvested.
Conclusion
Mr Juan Achurra, the farm owner said “In this field trial done on my farm, the Orego-Stim® Aquatract groups produced 3.5 tonnes harvest weight compared to the control group which produced 2 tonnes. Harvest weights per hectare were 913kg with Orego-Stim® Aquatract and 472kg per hectare in the control groups. These increased harvest weights gave me a 3:1 return on investment on the cost of Orego-Stim®, Aquatract.”
References
Ceniacua & Akvaforsk. 2002. Selective breeding of Litopenaeus vannamei in Colombia. Panorama Acuicola 7(2):30-31.
Chevassus, B. and M. Dorson, M. 1990. Genetics of resistance to disease in fishes. Aquaculture 85:83-107.
Henryon, M., A. Jokumsen, P. Berg, I. Lund, P.B. Pedersen, N.J. Olesen, and W.J. Slierendrecht. 2002. Genetic variation for growth rate, feed conversion efficiency, and disease resistance exists within a famed population of rainbow trout. Aquaculture 209:59-76.
Moss, S.M., R.W. Doyle, and D.V. Lightner. (2005) Breeding shrimp for disease resistance: challenges and opportunities for improvement. In: Diseases in Asian Aquaculture V. pp. 379-393. (P. Walker and R. Lester, eds.), Fish Health Section, Asian Fisheries Society, The Philippines.
Mr Juan Achurra (Farmer Owner), La Morenita, Via Al Puerto, Entrando por la estación Eneríque Enseñat, Región Coclé, Panama
World Health Organization. 1999. Joint FAO/NACA/WHO Study Group on food safety issues associated with products from aquaculture. WHO Technical Report Series, No 883.



