Biological features
Body elongate, compressed, with a deep caudal peduncle. Head pointed, with concave dorsal profile becoming convex in front of dorsal fin. Mouth large, slightly oblique, upper jaw reaching to behind eye; teeth villiform, no canines present. Lower edge of pre-operculum with a strong spine; operculum with a small spine and with a serrated flap above origin of lateral line. Lower first gill arch with 16 to 17 gillrakers. Scales large, ctenoid. Dorsal fin with 7 to 9 spines and 10 to 11 soft rays; a very deep notch almost dividing spiny from soft part of fin; pectoral fin short and rounded, several short, strong serrations above its base; dorsal and anal fins both have scaly sheaths. Anal fin rounded, with 3 spines and 7 to 8 short rays. Caudal fin rounded. Colour in two phases, either olive brown above with silver sides and belly (usually juveniles) or green/blue above and silver below. No spots or bars present on fins or body.
Profile
Historical background
Lates calcarifer, known as seabass in Asia and barramundi in Australia, is a large, euryhaline member of the family Centropomidae that is widely distributed in the Indo-West Pacific region from the Arabian Gulf to China, Taiwan Province of China, Papua New Guinea and northern Australia. Aquaculture of this species commenced in the 1970s in Thailand, and rapidly spread throughout much of Southeast Asia.
Among the attributes that make barramundi an ideal candidate for aquaculture are:
- It is a relatively hardy species that tolerates crowding and has wide physiological tolerances.
- The high fecundity of female fish provides plenty of material for hatchery production of seed.
- Hatchery production of seed is relatively simple.
- Barramundi feed well on pelleted diets, and juveniles are easy to wean to pellets.
- Barramundi grow rapidly, reaching a harvestable size (350 g – 3 kg) in six months to two years.
Today barramundi is farmed throughout most of its range, with most production in Southeast Asia, generally from small coastal cage farms. Often these farms will culture a mixture of species, including barramundi, groupers (Family Serranidae, Subfamily Epinephelinae) and snappers (Family Lutjanidae).
Australia is experiencing the development of large-scale barramundi farms that reflect the industrialised style of aquaculture seen in Europe. Where barramundi farming is undertaken outside the tropics, recirculation production systems are often used (e.g. in southern Australia and in the north-eastern United States of America).
Barramundi has been introduced for aquaculture purposes to Iran, Guam, French Polynesia, the United States of America (Hawaii, Massachusetts) and Israel.
Habitat and biology
Barramundi inhabit freshwater, brackish and marine habitats including streams, lakes, billabongs, estuaries and coastal waters. Barramundi are opportunistic predators; crustaceans and fish predominate in the diet of adults.
Spawning seasonality varies within the range of this species. Barramundi in northern Australia spawn between September and March, with latitudinal variation in spawning season, presumably in response to varying water temperatures. In the Philippines barramundi spawn from late June to late October, while in Thailand spawning is associated with the monsoon season, with two peaks during the northeast monsoon (August – October) and the southwest monsoon (February – June). Spawning occurs near river mouths, in the lower reaches of estuaries, or around coastal headlands. Barramundi spawn after the full and new moons during the spawning season, and spawning activity is usually associated with incoming tides that apparently assist transport of eggs and larvae into the estuary.
Barramundi are highly fecund; a single female (120 cm TL) may produce 30–40 million eggs. Consequently, only small numbers of broodstock are necessary to provide adequate numbers of larvae for large-scale hatchery production.
Larvae recruit into estuarine nursery swamps where they remain for several months before they move out into the freshwater reaches of coastal rivers and creeks. Juvenile barramundi remain in freshwater habitats until they are three–four years of age (60–70 cm TL) when they reach sexual maturity as males, and then move downstream during the breeding season to participate in spawning. Because barramundi are euryhaline, they can be cultured in a range of salinities, from fresh to seawater. When they are six–eight years old (85–100 cm TL), Australian barramundi change sex to female and remain female for the rest of their lives. Sex change in Asian populations of this species is less well defined and primary females are common.
Although some barramundi have been recorded as undertaking extensive movements between river systems, most of them remain in their original river system and move only short distances. This limited exchange of individuals between river systems is one factor that has contributed to the development of genetically distinct groups of barramundi in northern Australia, where there are six recognised genetic strains in Queensland, and a further ten in the Northern Territory and Western Australia.
Production
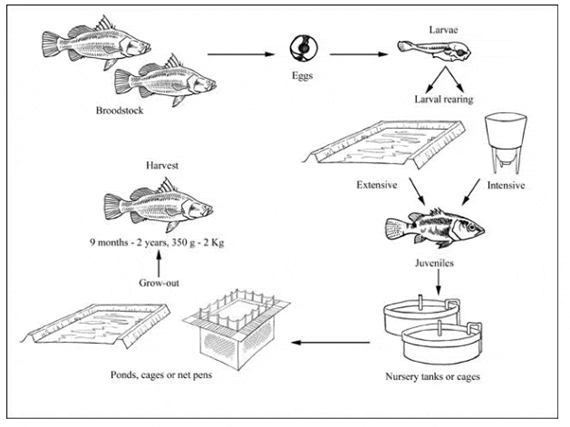
Production cycle of Lates calcarifer
Production systems
Seed supply
While barramundi fingerlings are still collected from the wild in some parts of Asia, most seed supply is through hatchery production. Hatchery production technology is now well established throughout the culture range of this species.
Rearing fingerlings
Barramundi broodstock are held in floating cages or in concrete or fibreglass tanks. They may be maintained in either fresh or seawater but must be placed in seawater prior to the breeding season to enable final gonadal maturation to take place. Barramundi show no obvious external signs of gonadal development and must be examined by cannulation to determine their gender and reproductive status, although milt can be expressed easily from male fish during the spawning season.
Barramundi broodstock are usually fed with 'trash' fish or commercially available baitfish. In order to improve the nutritional composition of the broodstock diet, and prevent diseases associated with vitamin deficiencies, a vitamin supplement may be injected into, or mixed with, the baitfish prior to feeding.
Asian barramundi have been induced to spawn by manipulation of environmental parameters (salinity and temperature) to simulate the migration to the lower estuary, and the tidal regime there at the time of natural spawning. The same techniques have proven unsuccessful with Australian populations of barramundi, which generally require hormonal induction to spawn. Barramundi have been successfully spawned using a range of hormones at various doses, which were administered by techniques including injection, slow-release cholesterol pellets and osmotic pumps. Induced spawning of barramundi is now generally carried out using the leuteinising hormone-releasing hormone analogues (LHRHa) (Des-Gly10)D-Ala6,Pro9-LH-RH ethylamide and (Des-Gly10)D-Trp6, Pro9-LH-RH ethylamide.
Pre-spawning behaviour involves the male fish pairing with a female and rubbing its dorsal surface against the area of the female's genital papilla, erecting its fins and 'shivering'. In the absence of such displays, egg release may occur but they are not fertilised. Spawning occurs 34–38 hours after injection, usually around dusk, and may be accompanied by violent splashing at the water surface. Barramundi will often spawn for up to five consecutive nights.
At spawning, the sperm and eggs are released into the water column and fertilisation occurs externally. Barramundi eggs are 0.74–0.80 mm in diameter with a single oil droplet of 0.23–0.26 mm diameter. The eggs are collected from spawning tanks using fine mesh (around 300 µm) egg collection nets through which tank water is diverted. If barramundi are spawned in cages, the cages are lined with a fine mesh 'hapa' net that retains the eggs inside the cage, enabling their later removal to the hatchery.
Fertilised eggs undergo rapid development and hatching occurs 12–17 hours after fertilisation at 27–30 °C. Newly hatched larvae have a large yolk that is absorbed rapidly over the first 24 hours after hatching, and is largely exhausted by 50 hours after hatching. The oil globule is absorbed more slowly and persists for about 140 hours after hatching. The mouth and gut develop the day after hatching (day two) and larvae commence feeding from 45–50 hours after hatching.
Hatchery production
Barramundi are generally reared using 'green water' intensive techniques, in circular or rectangular concrete tanks or in circular canvas tanks up to 26 m³ capacity. A microalgal culture (usually Tetraselmis spp. or Nannochloropsis oculata) is added to the rearing tanks at densities ranging from 8–10×10³ to 1–3×105 cells/ml. Intensively reared barramundi are fed on rotifers (Brachionus plicatilis) from day two (where day one is the day of hatching) until day 12 (or as late as day 15), and on brine shrimp (Artemia sp.) from day eight onwards. Both rotifers and brine shrimp fed to barramundi are cultured on microalgae or commercial enrichment products to increase levels of highly unsaturated fatty acids. The freshwater cladocerans Daphnia and Moina have been used to supplement, or replace, brine shrimp as prey for intensively reared barramundi larvae. Overall survival for intensively-reared barramundi larvae from hatching to about 10 mm TL generally ranges from 15–50 percent. More recently, compounded microdiets have been used to partly or totally replace brine shrimp in the intensive larval rearing of barramundi.
Barramundi fingerlings are also produced using extensive (pond-based) rearing procedures. Pond areas used for the extensive larval rearing of barramundi range from 0.05 to 1 ha and may be earthen or plastic lined. They are relatively shallow (<2 m) to promote maximum production of phytoplankton and to prevent stratification. Larval rearing ponds are managed through the application of inorganic and organic fertilisers to produce a 'bloom' of suitable zooplankton concomitant with the introduction of the newly hatched barramundi larvae. Barramundi larvae are stocked at densities of 400 000–900 000/ha. Continued pond management focuses on supporting adequate zooplankton populations for the developing larvae, and ensuring that water quality criteria are maintained. Barramundi are harvested from the ponds when they reach 25 mm TL or greater (about three weeks after stocking), and are then transferred to nursery tanks. Survival of extensively reared barramundi averages about 20 per cent, but is highly variable, ranging from zero to 90 percent. Production rates of up to 640 000 fish/ha have been achieved in extensive rearing.
Nursery
Barramundi juveniles (1.0–2.5 cm TL) may be stocked in floating or fixed nursery cages in rivers, coastal areas or ponds, or directly into freshwater or brackishwater nursery ponds or tanks. The fish are fed on minced trash fish (4–6 mm) or on small pellets. Vitamin premix may be added to the minced fish at a rate of 2 percent. This nursery phase lasts for 30 to 45 days; once the fingerlings have reached 5–10 cm TL they can be transferred to grow-out ponds.
Cannibalism can be a major cause of mortalities during the nursery phase and during early grow-out because barramundi will cannibalise fish of up to 61–67 percent of their own length. Cannibalism may start during the later stages of larval rearing and is most pronounced in fish less than about 150 mm TL; in larger fish, it is responsible for relatively few losses. Cannibalism is reduced by grading the fish at regular intervals (usually at least every seven–ten days) to ensure that the fish in each cage are similar in size.
Ongrowing techniques
Most barramundi culture is undertaken in net cages. Both floating and fixed cages are used; these range in size from 3×3 m up to 10×10 m, and 2–3 m depth. In Australia and the United States of America, a number of barramundi farms have been established using recirculation freshwater or brackishwater systems with a combination of physical and biological filtration. These farms may be located in regions where barramundi could not otherwise be farmed because of consistently low temperatures (southern Australia, north-eastern United States of America). The major advantage of such culture systems is that they can be sited near to markets in these areas, thus reducing transport costs for the finished product.
The stocking densities used for cage culture generally range from 15 to 40 kg/m³, although densities may be as high as 60 kg/m³. Generally, increased density results in decreased growth rates, but this effect is relatively minor at densities under about 25 kg/m³. Barramundi farmed in recirculation production systems are stocked at a density of about 15 kg/m³.
Barramundi are also farmed in earthen or lined ponds without cages; a technique known in Australia as 'free ranging'. Juvenile barramundi (20–100 g) are cultured in brackishwater ponds at 0.25–2.0 fish/m². In Asia, barramundi may be polycultured in brackishwater ponds with tilapia (Oreochromis spp.) as a food source.
Feed supply
Most barramundi are now fed on compounded pellets, although 'trash' fish is still used in areas where it is cheaper or more available than pelleted diets. Barramundi fed 'trash' fish are fed twice daily at 8–10 percent body weight for fish up to 100 g, decreasing to 3–5 percent body weight for fish over 600 g. Vitamin premix may be added to the trash fish at a rate of 2 percent, or rice bran or broken rice may be added to increase the bulk of the feed at minimal cost. Food conversion ratios (FCRs) for barramundi fed on trash fish are high, generally ranging from 4:1 to 8:1.
Barramundi fed pellets are generally fed twice each day in the warmer months and once each day during winter. Larger farms may use automatic feeder systems, though smaller farms hand-feed. Barramundi have achieved FCRs of 1.0–1.2:1 under experimental conditions, but in commercial farm conditions FCRs of 1.6–1.8:1 are usual. FCR varies seasonally, often increasing to over 2.0:1 during winter.
Harvesting techniques
For barramundi farmed in cages, harvesting is relatively straightforward, with the fish being concentrated into part of the cage (usually by lifting the net material) and removed using a dip net. Harvesting barramundi 'free-ranging' in ponds is more difficult, and requires seine-netting the pond or drain harvesting.
Handling and processing
After harvesting, the barramundi are placed in an ice slurry to kill them humanely and preserve flesh quality. In Australia, most farms do not process the fish, but sell them 'gut in'. Some larger Australian farms have processing facilities to process fillet product from larger (2–3 kg) fish. Fresh barramundi is generally transported packed in plastic bags inside styrofoam containers with ice. There is a limited market for live barramundi in Australia and in Southeast Asia. Fish are usually transported live in tanks by truck.
Production costs
Economic models of barramundi farming in Australia have estimated the break-even cost for a small (50 tonnes/yr) Australian farm to be AUD 9.25/kg (USD 6.90/kg), and the break-even cost for a 200 tonnes/yr farm at AUD 6.90 (USD 5.1). Larger farms (>1 000 tonnes/yr) are able to take advantage of economies of scale and their production costs are likely to be around AUD 6–7/kg (USD 4.50–5.25/kg). In contrast, barramundi farms in Thailand can produce fish for USD 1.90/kg. Economic modelling of Australian barramundi farms indicated that profitability was particularly sensitive to price, with a decrease of AUD 1.00 (USD 0.75) resulting in an 80 percent decrease in equivalent annual return.
Farm-level economic spreadsheet models for barramundi farming are available from the Queensland Department of Primary Industries and Fisheries (http://www.dpi.qld.gov.au).
Diseases and control measures
The major diseases to which barramundi are susceptible are listed in the following table.
In some cases antibiotics and other pharmaceuticals have been used in treatment but their inclusion in this table does not imply an FAO recommendation.
| DISEASE | AGENT | TYPE | SYNDROME | MEASURES |
|---|---|---|---|---|
| Viral nervous necrosis (VNN) | Lates calcarifer encephalitis virus (LcEV) – a betanodavirus | Virus | Pale or dark colouration; erratic swimming behaviour; spiral swimming; bloating; 'fainting'; extensive vacuolation of the brain & spinal cord; generally encountered during hatchery phase | Screening of broodstock; low larval rearing densities; optimal larval nutrition; improved broodstock nutrition; improved hatchery hygiene |
| Lymphocystis | Lymphocystis virus | Virus | Wart-like growths on skin & fins; generally only fatal if infection severe & associated with very poor environmental conditions | Removal of infected fish; improved environment |
| Vibriosis | Vibrio harveyi; Vibrio spp. | Bacteria | Marine fish with darkening; lethargy; anorexia; reddened ulcerations on body; reddened abdominal fluid; associated with nursery systems, poor environment & skin trauma | Improved environment; antibiotic treatment |
| Bacterial haemorrhagic septicaemia | Aeromonas hydrophila; AAeromonas sobria; Aeromonas caviae; Aeromonas spp.; Pseudomonas sp. | Bacteria | Freshwater fish with irregular reddened skin ulcerations; lethargy; anorexia; reddened abdominal fluid; pale gills; associated with poor environment & skin trauma | Improved environment; antibiotic treatment |
| Integumentary bacteriosis | Aeromonas sobria; Aeromonas hydrophila; Vibrio harveyi; Vibrio alginolyticus | Bacteria | Irregular reddened skin ulcerations; loss of scales; associated with poor environment & skin trauma | Improved environment; increased water exchange |
| Streptococcosis | Streptococcus iniae | Bacterium | Darkened fish; anorexia; pale gills; reddened abdominal fluid; reddened abdominal organs & inner wall | Antibiotic treatment; vaccination |
| Columnaris disease | Flavobacterium columnare; Flavobacterium johnsoniae; & Flavobacterium sp. (gliding forms) in freshwater Tenacibaculum marinimum in seawater |
Bacteria | Pale skin patches on dorsal surface behind dorsal fin & on caudal peduncle; lethargy; most commonly occurs in nursery phase; in older juveniles a mouth form with erosion of skin around upper & lower jaws has been seen; associated with overstocking, tank rearing, poor hygiene & skin trauma | Treatment in potassium permanganate or copper baths may help in early disease; antibiotic treatment |
| Bacterial gill disease | Various bacteria, Flavobacterium spp., Cytophaga spp. | Bacteria | Swimming at water surface; gulping; rapid opercular movement; excess mucus on gills; white patches on gills; most commonly occurs in nursery phase | Improve water quality; treatment with salinity reversal, potassium permanganate or quaternary ammonium baths; increase water exchange; reduce stocking density |
| Bacterial peritonitis | Various Gram-negative & Gram-positive bacteria including Vibrio harveyi & Aeromonas hydrophila | Bacteria | Darkened fish; lethargy; swollen abdomen; adhesions & bad smelling fluid in abdomen; abdominal fistulas; more common in recirculation systems | Cull affected fish; antibiotic treatment |
| Bacterial enteritis | Various Gram-negative bacteria | Bacteria | Acute disease in intensive larval rearing systems; anorexia; pin heads; darkened fish & death | Cull affected larval batch |
| Fin and tail rot | Aeromonas spp.; Pseudomonas spp.; Vibrio spp.; Flavobacterium spp.; Cytophaga spp. | Bacteria | Erosion of soft tissue in fins and tail; may extend to involve entire tail & caudal peduncle | Improve environment; reduce stocking density |
| Epitheliocystis | Epitheliocystis organism – a Chlamydia | Bacterium | Swimming at water surface; rapid opercular movements; disease rare but seen in marine fish & in recirculation systems | None known |
| White spot | Ichthyophthirius multifiliis in freshwater Cryptocaryon irritans in marine |
Protozoa | 'Flashing'; rubbing skin on surfaces; anorexia; swimming at water surface; white spots on skin & fins | Treatment with salinity reversal, formalin baths or combinations; treatment in copper bath for marine fish |
| Chilodonelliasis | Chilodonella spp.; Chilodonella hexasticha | Protozoa | Swimming at water surface; rapid opercula movement; flared opercula; seen in poor environmental conditions & in weakened fish | Treatment with salt, formalin or potassium permanganate bath or combinations |
| Trichodiniasis | Trichodina complex spp. | Protozoa | Swimming at water surface; rapid opercular movements; excess gill mucus; typically follows cold water temperatures, high organic loads & high stocking densities | Increase water exchange; treatment with salt or formalin bath |
| Ichthyobodosis (costiasis) | Ichthyobodo necator | Protozoa | 'Flashing'; rubbing skin on surfaces; opaque patches on skin; raised scales; swimming at water surface; rapid opercular movements; flared opercula | Treatment with salinity reversal; formalin or potassium permanganate bath |
| Piscinoodiniasis | Piscinoodinium sp. | Protozoa | Found in freshwater: In young fish opaque patches or a greenish discolouration of the skin; patches of skin lifting of surface & ulcers In older fish rapid opercular movements; excess gill mucus; dark green gill colour | Treatment with salt bath |
| Amyloodiniasis | Amyloodinium ocellatum | Protozoa | Found in marine conditions: In young fish opaque patches or a green discolouration of the skin; patches of skin lifting of surface & ulcersIn older fish rapid opercular movements; excess gill mucus; dark green gill colour More common in broodstock and in raceways; associated with low water temperatures or rapid drops in temperature | Treatment with freshwater, copper, formalin or hydrogen peroxide bath |
| Red sore disease | Epistylis sp. | Protozoa | Skin ulcers in freshwater pond fish; raised fluffy surface & secondary bacterial infections | Reduce organic levels in water; treatment with formalin bath |
| Gill fluke | Diplectanum sp.; Dactylogyrus sp. | Monogean trematodes | Rapid opercular movements; anorexia; white areas on gills | Treatment with salinity reversal, formalin, organo-phosphate or praziquantel bath |
| Skin fluke | Neobenedinia melleni; Gyrodactylus spp. | Monogean trematodes | Marine fish with opaque cornea; white patches on skin; skin ulcers; associated with high salinity & cool water temperatures | Treatment in freshwater or hydrogen peroxide bath |
| Myxosporidiosis | Henneguya sp.; Kudoa sp. | Spore-forming protozoa | Disease uncommon but histologically spore cysts seen in gill filaments (Henneguya sp.) & brain (Kudoa sp.) | None known |
| Microsporidiosis | Pleistophora sp. | Spore-forming protozoa | Raised lumps on skin; soft white nodules in muscle | None known |
| Integumentary mycosis | Saprolegnia spp.; Achlya spp. | Fungi | Raised, fluffy growths on skin & fins; associated with low water temperatures & skin trauma | Salinity reversal and formalin baths; do not handle fish when water temperatures low |
| Branchiomycosis | Brachiomyces sp.; Achlya spp. | Fungi | Swimming at water surface; rapid opercular movements; white & red patches (mottled appearance) on gills; associated with cold water temperatures & high organic loads | No treatment known; reduce organic load & increase water exchange |
| Fish louse | Argulus sp. | Copepod | Disc-shaped parasite visible on skin; red foci; darkening | Treatment in organophosphate bath |
| Anchor worm | Lernaea sp. | Copepod | Thin body of female parasite visible on skin with small red ulcer where parasite penetrates skin | Treatment in organophosphate bath |
Statistics
Production statistics
Annual barramundi production has been relatively static since 1998, at ~20 000–27 000 tonnes. Thailand is the major producer, with about 8 000 tonnes/yr since 2001. Indonesia, Malaysia and Taiwan Province of China are also major producers. The global average value of farmed barramundi was USD 3.80/kg in 1994 and rose to USD 4.59/kg in 1995 but had fallen to USD 3.92 by 1997. Since then it has been around USD 3.7/kg except for 2002, when it fell markedly to below USD 3.0/kg.
Market and trade
In Asia, most barramundi are marketed at 500–900 g, although small numbers of larger fish (1–3 kg) are also sold.
In Australia, there are two main products from farmed barramundi: 'plate size' and fillet product. 'Plate size' fish range from 350–500 g, although larger (banquet) fish may be up to 800 g. Fillet product fish are generally in the range of 2 to 3 kg.
There has been little effort put into developing value-added products for barramundi. In Australia, there are a few suppliers of smoked barramundi. Throughout its cultured range, live barramundi are sold to restaurants that specialize in live seafood products, but this is a relatively small proportion of the total market for barramundi.
There is relatively little import and export of barramundi – most are consumed locally. One exception is the culture of barramundi in recirculation production systems in the United States of America, with fingerlings exported by air from Australia.
The Australian Barramundi Farmers Association has adopted product quality standards to address the issue of highly variable product quality in the Australian market.
Status and trends
There has been considerable research effort expended on barramundi aquaculture since the 1970s and this has contributed to the reliability and cost-efficiency of production of this species globally. Because of the mature nature of the barramundi aquaculture industry, there is comparatively little on-going research. Most research institutes that were involved in developing barramundi farming technologies have moved on to other species, such as groupers. One major area where there is a recognized need, but to date little effort, is in genetic selection programmes targeting faster growth and disease resistance.
Another major area of research, that incorporates the overall marine finfish aquaculture industry in Asia, is the assessment of the environmental impacts of cage aquaculture. The rapid expansion of marine finfish aquaculture in Asia is having a range of impacts (see Main Issues below). Although there is some research into the amelioration of these impacts and improved planning for coastal aquaculture development, the development of planning and implementation frameworks to ensure the sustainability of coastal cage aquaculture remains a challenge for many countries.
In most countries where it is farmed, there has been relatively little market development for barramundi. In Asia, barramundi is a relatively inexpensive product and most farmer interest is now on higher-value species, such as groupers.
The major topics that need addressing are:
- Selective breeding programmes for faster growth and improved disease resistance need to be established.
- Improved marketing of barramundi, both in producer countries and internationally, is essential to expand market demand.
- Development of value-added products is necessary, in order to expand market demand in industrial countries.
Main issues
Environmental impacts associated with marine finfish cage aquaculture derive mainly from nutrient inputs from uneaten fish feed and fish wastes. For example, studies carried out in Hong Kong indicate that 85 percent of phosphorus, 80–88 percent of carbon and 52–95 percent of nitrogen inputs (from 'trash' fish) to marine finfish cages may be lost through uneaten food, faecal and urinary wastes. These nutrient inputs, although small in comparison with other coastal discharges, may lead to localised water quality degradation and sediment accumulation. In severe cases, this 'self pollution' can lead to cage farms exceeding the capacity of the local environment to provide inputs (such as dissolved oxygen) and assimilate wastes, contributing to fish disease outbreaks and undermining sustainability.
Responsible aquaculture practices
In Australia, an environmental code of practice for freshwater finfish aquaculture has been prepared and adopted by the Aquaculture Association of Queensland; this has not yet been adopted by the Australian Barramundi Farmers Association, but is likely to provide the basis for a similar code of practice for barramundi farmed in freshwater ponds.
The Australian Barramundi Farmers Association has developed and adopted a Code of Practice for Post-Harvest Handling of Farmed Barramundi; this is aimed at improving product quality through best post-harvest practices.
A generic code of practice for holding live seafood products is the 'Guidelines on welfare of fish and crustacea held in live holding systems for human consumption' (see useful web sites under related links below).
Barramundi farming should also adhere to the general principles laid down in the FAO Code of Conduct for Responsible Fisheries and the relevant Technical Guidelines.
April 2010





