In 2011 more than 500 million litres of Whisky were produced in the United Kingdom (SWA, 2011). It has been reported that for every litre of alcohol distilled, on average 8 to 15 litres of effluents are generated (Mohana et al., 2009). These co-products – which include liquid and solid components – contain important amounts of proteins that are currently underutilised and perceived as a problem rather than opportunity for the Whisky Industry.
The Norwegian and Scottish Aquaculture Industry, with a growing demand for proteins to satisfy the nutritional requirements of their fish stocks, could take advantage from the proteins available from Malt (MW) and Grain Whisky (GW) co-products.
By reducing the reliance on wild fish and imported protein sources (e.g. soybean meal), the Aquaculture Industry could benefit economically and improve substantially the sustainability of their protein supply chain. An understanding of the nutritional, chemical and physical properties of the Whisky co-products; the development of sustainable methods for protein recovery - suitable to both whisky and aquaculture needs – and the economics behind the process and product are essential to ensure a sustainable large scale protein supply to the Aquaculture Feed sector. The present work focuses on the nutritional properties of these co-products and highlights of the protein recovery processes (i.e. protein extraction and concentration) developed by Horizon Proteins, a research group from Heriot-Watt University (Scotland).
Materials and Methods
Nutritional and Physical Properties Liquid and solid co-products samples from MW and GW origin across several Scottish distilleries were collected and analysed for physical, chemical and nutritional properties. Physical properties analyzed included pH (liquid samples) and dry matter content of liquid and solid samples (oven dried at 105°C, 24 hours). Liquid samples were centrifuged at 5000g for 15 minutes. Supernatants and solid fractions were subsequently analysed for the content of protein, trace metal,
phosphate and some known anti-nutritional compounds.
Soluble Protein was determined by the Bradford assay and the Kjeldahl method was used for Total Protein Content for liquid and solid samples. Trace metal content was detected by flame atomic absorption spectroscopy (FAAS). Total phosphate and anti-nutritional compounds were determined by spectrophotometric methods.
Protein Extraction Methods
Protein was extracted from solid particles (yeast) contained in the liquid co-products samples using a High Pressure Homogeniser (HPH), model APV-1000 from APV Systems. Samples were exposed to a range of pressures with recirculation. Homogenised samples were collected every 5 minutes for Protein content analysis (Bradford Assay).
Protein Concentration Methods
Ion Exchange Chromatography (IEC) techniques were used to concentrate protein in liquid samples. Liquid samples were previously centrifuged (5000g, 15 minutes) and run through different 1 ml column media (Hi-Trap Capto Q and Hi-Trap Capto S, GE-Healthcare) at different process conditions (flow rate and loading time) in a liquid chromatography system (Äkta Avant 150, GE-Healthcare). Elution was performed under several pH and buffer conditions.
Results and Discussion
Protein in liquid co-products could be up to 40% (dry matter basis) and similar values could be found in solid streams.
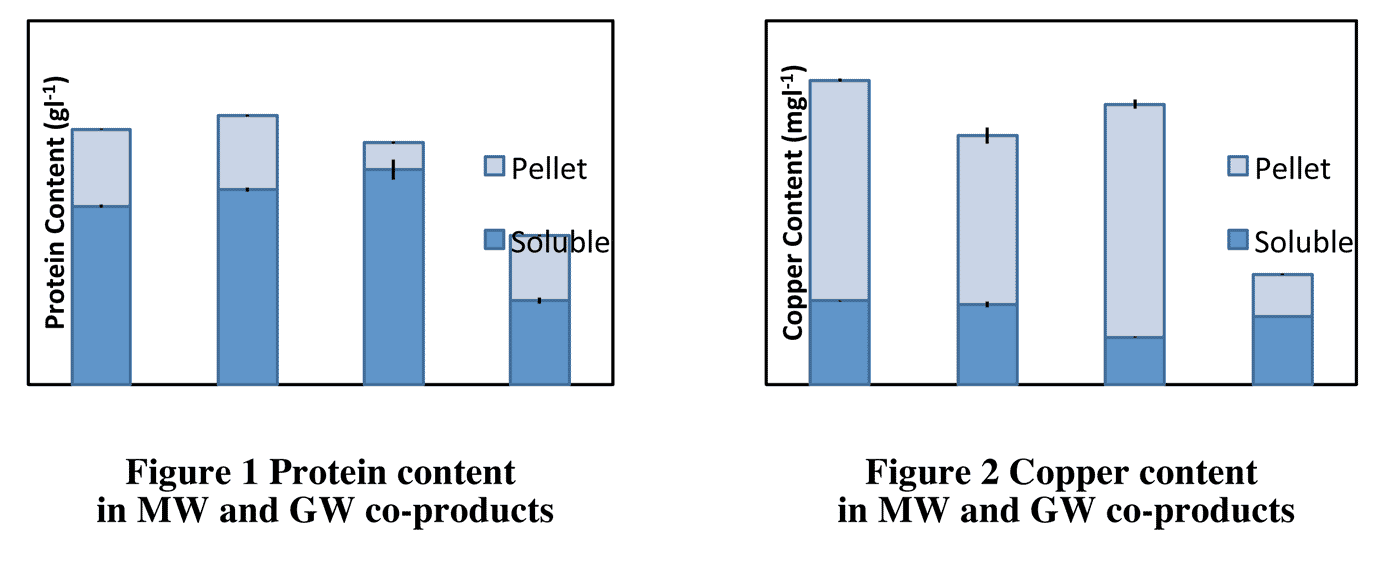
However, differences close to 50% in protein content (gl-1) between MW and GW sources were detected in the samples analysed (Figure 1). Variations in protein partition were also discovered. In some cases around 70% of the protein content was found in the soluble fractions of the liquid co-products. Characterisation of co-products for metal content confirmed that most of the copper of the liquid co-products is bound to the solid fraction (Figure 2).
Protein extraction experiments (HPH) resulted in an increment of soluble protein by nearly 5 times. Protein concentration methods utilised during this work (IEC) proved to be satisfactory for liquid co-products and high levels of protein concentration were achieved during the trials.
Conclusions
Whisky co-products could offer a sustainable supply of suitable protein to the Aquaculture Industry. The proteins in these co-products are nutritionally comparable to proteins used in available Fish Feed ingredients. Potential protein volumes from Whisky co-products could be enough to satisfy the Scottish Salmon Farming and achieve substantial reductions in unsustainable protein sourcing. There is a need to identify appropriate and sustainable techniques to obtain these proteins on an industrial scale.
Protein recovery using methods developed by Horizon Proteins could have much more financial viability than traditional treatment technologies for Whisky Co-products. The savings are not only shown economically, but through the significant reduction in energy requirements in processing, reducing the global warming impact of the overall process.
October 2013




