Introduction
The timing of their migratory spawning behavior (e.g., upriver migration from the ocean), presence of ripe fish in the spawning grounds, and external appearance of the brood fish have traditionally been used to assess the stage of maturity and specific time of spawning. However, assessment of ready to spawn (ripe) sturgeon based simply on behavioral and external sex-limited characteristics can be misleading especially in domestic stocks that are raised under aquaculture conditions, as they are not exposed to natural conditions that promote sexual maturity and serve as spawning cues. Selection of female fish for spawning without proper assessment of their stage of egg maturation often results in partial or no ovulation, or ovulation occurs but with low egg fertility or low embryo survival. Many factors influence ovulation and spawning success in farmed sturgeon, from temperature and light regimes, husbandry methods, handling and transport, induced spawning protocols, and genetic make-up of the stock. However, relatively simple field and laboratory tests can be used to assist in predicting ovulatory response in female sturgeon. The simplest and most reliable indicators that a female is ready to spawn are egg size and the position of the egg nucleus in the cytoplasm, referred as the egg polarization index or PI.
Principle
The most visible feature in the process of egg (oocyte) formation (oogenesis) is the enormous growth of the egg due to the continuous accumulation of yolk and other nutrients from the blood. After complete yolk deposition the eggs of sturgeon achieve their maximum size, which is a good indicator of spawning readiness. Egg size has been demonstrated to be a heritable trait in fishes, and there is a positive correlation between egg size and growth; although it is influenced by female size and age, food supply and quality, and other environmental factors.
Sturgeons show a clear evolutionary relationship between egg size and species, with each species having a specific egg size range, exhibited both in wild and domestic stocks. For example, Green sturgeon (Acipenser medirostris) has very large eggs, ranging from 4.0 to 4.7 mm in diameter, compared to 2.0 - 2.8 mm for Sterlet (A. ruthenus), one of the smallest sturgeon eggs. Typical egg sizes for species in the traditional sturgeon fisheries are 3.6 - 4.3 mm for Beluga (Huso huso), 3.2 - 3.8 mm for Osetr (A. gueldenstaedti), and 2.7 - 3.2 mm for Sevruga (A. stellatus). Egg sizes for commonly cultured sturgeon species are 3.2 - 4.0 mm for White sturgeon (A. transmontanus), and 2.4 - 3.0 mm for Siberian sturgeon (A. baerii).
In the ovary, follicular cells that facilitate yolk accumulation surround individual eggs. Between the egg membrane and follicular cells, is the egg chorion, a tough envelope of variable thickness that protects the developing embryo during incubation. As the egg grows in size, so does its nucleus that is then referred to as a germinal vesicle (GV); the egg is now reaching maturity. During each ovarian cycle, the eggs are in an arrested stage of cell and nuclear division (i.e., meiosis is arrested) for a prolonged period of time, while the eggs accumulate yolk and grow to a determined size; genetic information is also transcribed during this time. For the first ovarian cycle the eggs in female sturgeon are arrested in meiosis for many years (typically 2-6 years) until the female reaches puberty. Thereafter, each ovarian cycle may take from a few months to several years.
Spawning success is primarily influenced by the stage of egg maturation. Once a species-specific determined egg size is reached, the egg enters final maturation and resumes meiosis. At the same time the GV begins to migrate to the edge of the egg where it undergoes germinal vesicle breakdown or GVBD. If the eggs are sampled at progressive time intervals, the position of the GV clearly changes from the center of the egg to the periphery, near the animal pole. The oval-shaped egg has an animal and vegetal pole ( Figure 1 ). The animal pole region (AP) contains the fine, typically whitish-colored yolk (F-Yolk), while the vegetal pole (VP) has the larger, yellowish-colored course yolk platelets (C-Yolk). When the GV is near the edge of the egg plasma membrane, just below the chorion, the female is ripe and ready to spawn. The migration of the GV to the periphery of the egg may take from a few days to weeks, and even months, dependent primarily on water temperature. Most cultured sturgeon are reared at water temperatures 4-6 °C above optimal spawning temperature to maximize growth. The preferred water temperatures for spawning sturgeon ranges between 14 and 18 °C, depending on species. Months before spawning, selected female (and male) broodstock should be kept at cooler water temperatures (preferably 4-5 °C below spawning temperature). Prior to spawning water temperatures are slowly increased to optimal spawning temperatures, to mimic what occurs during the spring warming in rivers where sturgeon spawn naturally.
Materials
- For fish handling: tube-net, stretcher and sawhorses, freshwater source, tag or ID system.
- For the gonad biopsy: surgical gloves, catheter of flexible tubing at least 4 mm ID or modified trocar, #3 scalpel handle and #10, or #15 blades, Adson-Brown tissue forceps 7x7 teeth, Olsen-Hegar needle holder scissors combination, absorbable sutures (e.g., Vicryl-polyglactin 910, size 0 or 1 with OS-6 or 4 cutting needle; or PDS II-polydioxanone, size 1 with CP-1 cutting needle), 1% iodine solution, sterile wipes, alcohol for cleaning/sterilizing instruments in between samplings, and a small "sharps" container for the used blades and suture needles.
- For the egg samples: 25 mL plastic vials with caps, Ringer's solution in 1 L plastic bottles, two holding coolers with reusable cold gel packs or wet ice, to maintain Ringer's temperature and to transport the vials of eggs at 14-18 °C, balance (or access to one) and accessories to weigh chemicals (NaCl 6.5 g, NaHCO3 2 g, CaCl2 300 mg, and KCl 250 mg) to make Ringer's solution when added to 1 L of distilled or de-ionized water.
- For PI determination: disposable plastic transfer pipettes (to handle/transfer eggs), 20 mL glass beakers, boiling stones, aluminum foil, hot plate, oven mitts, timer, 25 mL plastic vials with caps, vial rack, ice tray/bucket, 10% formalin fixative (9 parts water : 1 part formaldehyde), tools to handle and cut the eggs including delicate Adson-Brown forceps and double-edge razor blades (these are the thinnest), cutting surface (e.g., Petri-dish bottom), Ringer's stock solution.
- Miscellaneous: data sheets or notebook (preferably waterproof), pens/pencils, container for waste, tackle box or similar to store the surgical tools, sutures, etc., small container to hold the surgery tools in disinfectant, hand and/or paper towels, small table to place the surgery tools, sutures, and other sampling materials.
Biopsy Procedure
Females are first sampled approximately one month prior to the earliest expected spawning and then up to two or three times more, as individual females mature and spawn at different times. The spawning season typically lasts about three months but can be extended if females are held at colder water temperatures (6-7 °C below spawning temperature). Females should be captured individually using a tube-net and then placed ventral side up into a stretcher. Immediately upon positioning the female in the stretcher a supply of fresh, oxygenated water is gently placed in or just outside the mouth to irrigate the gills. Because the entire biopsy procedure only takes 2-3 minutes and since large ripe females tend to remain calm while ventilating the fresh water, no anesthetic is used. Also the fasting of fish prior to this procedure is not required.
To obtain a sample of eggs, a small incision (6-8 mm) is made approximately 3 to 5 ventral scutes anterior from the pelvic fin and 2.5-5.0 cm off the ventral mid-line using a scalpel. The ideal incision site is made lateral to the mid-line, enough so that you cut through some well vascularized muscle tissue that will enhance the healing process. The incision has to be just large enough to insert a flexible tubing (see Materials) to serve as a cannula or catheter. A modified solid trocar, to penetrate the skin into the body cavity, can also be used as an alternative method to obtain a sample of the eggs. Near the tip of the trocar a slit is made, long and deep enough to collect several dozen eggs at a time. The abdominal incision is closed using an absorbable synthetic suture (see Materials), using a single interrupted or cross-stitch. It should be noted that excessive swabbing of the incision area with a disinfectant is not recommended because some surgical scrub solutions may damage the fish's skin and heavy swabbing will remove the mucus, which has some antimicrobial activity. If deemed necessary, a gentle swab of excess mucus from the incision site with a sterile gauze pad and a little 1% iodine solution is all that is needed.
Approximately 20-30 eggs are sampled from each female and are placed into a 25 mL plastic vial containing modified Ringer's solution (see Materials). Vials are convenient because they can be capped to keep the eggs from spilling-out during transport. The eggs are rinsed 2-3 times with fresh Ringer's solution to remove any yolk and loose tissue from broken eggs, blood, and fatty tissue. After rinsing, enough fresh Ringer's solution is added to completely cover the eggs. Keep the eggs cool (14-18 °C) by placing them in an ice chest, containing wet or gel ice, but do not place them directly on ice. Use a float rack or similar device to keep the eggs elevated about 3-6 cm above the ice, and take them to the lab or processing area as soon as possible.
Determination of Egg Polarization Index (PI)
In the lab the eggs are placed into individual 20 mL glass beakers and labeled with the female identification number. Ringer's stock solution is added to each beaker, to a total volume of 15-20 mL, and the eggs boiled gently for about 5 minutes. A piece of marble gravel (or boiling stone) should be added to each beaker and all the beakers covered with a sheet of aluminum foil, as the eggs will occasionally "pop-out" of the beaker during boiling. After boiling, the eggs are chilled by placing the beakers directly on crushed ice for 15-30 minutes. At this time the eggs can be cut and egg measurements taken. Storing the eggs in 10% buffered formalin overnight will make cutting much easier, because some very ripe females have slightly soft eggs, even after chilling. These eggs become firmer and easier to cut after storage in buffered formalin.
The eggs are sectioned along the longest axis (the animal-vegetal axis) usually recognized by the oval shape of the freshly collected egg. However, the boiling, chilling and fixing of these eggs tends to result in a more rounded shape. Fortunately, in most cases the animal pole can be distinguished by the coloration (rings and/or a white spot of variable size). The individual eggs are held with forceps and bisected with a razor blade. Turn both halves section side up and they should be mirror images of each other if sectioned properly ( Figure 1 ). The presence of the GV is observed by focusing a light beam (e.g., dissecting microscope with fiber-optic illuminator) on the surface of either half. An important factor in determining the PI is correct sectioning of the eggs across their animal-vegetal axis. The eggs need to be bisected in such a way that there are two equal-size halves, with equal size GV in both halves. If you angle your cut and/or miss the animal-vegetal pole axis the PI measurement will not be accurate.
To calculate the PI, first measure the longest diameter, along the animal-vegetal axis. Next measure the distance from the outer edge of the GV to the egg membrane or bottom of the egg chorion (the thin black pigment layer). The PI is calculated by dividing the distance of the nucleus to the periphery of the egg by the diameter of the egg. Select females with PI scores of less than 0.10 for spawning induction, but preferably those females with egg polarization indices of 0.06-0.08.
For making the egg measurements there are several tool options from a simple dissecting microscope equipped with an eyepiece micrometer, to a digital stereoscope and computerized image system. A zoom dissecting scope is preferred for the capability to gradually increase the magnification so that the entire egg can be viewed at the highest magnification possible. Dissecting scopes with pre-set magnifications (e.g. 5x, 10x, 20x, 40x) are also used but sometimes sturgeon eggs are too small for accurate measurements at the lower magnifications and too large at the higher magnifications. A trinocular dissecting microscope with an attached video camera and a TV/video screen can be used to view the sectioned egg, and then a ruler can be used to measure the distances to calculate the PI. A similar system using a digital camera or computer microscope connected to a laptop can be used and the measurements made of the egg image directly on the computer screen or by using a measuring or imaging software (e.g., Cell Profiler ). Whatever equipment is used, remember to be consistent at what magnification your eggs are observed, so that over time you can become experienced enough to estimate the PI by sight alone, a skill that is especially useful if you are in the field.
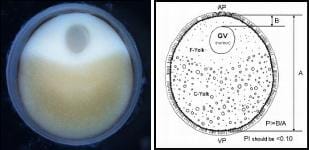 |
| Figure 1. A photograph and drawing of a sturgeon egg (oocyte) depicting the clearly polarized nucleus or GV (off-center), and defined animal (AP) and vegetal (VP) poles. Segregation of the fine and course yolk granules helps to orientate the position of the two poles; the GV is embedded in the cortical cytoplasm of the animal pole region. The egg polarization index or PI = B/A; where A is diameter of the egg, excluding the chorion, and B is distance from the top of the GV to the plasma membrane of the egg; see diagram. For spawning, select females with egg polarization indices (PI) of less than 0.10. |
Footnotes
- This document is FA153, one of a series of the Fisheries and Aquatic Sciences Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Original publication date December 2007. Visit the EDIS Web Site at http://edis.ifas.ufl.edu.
- Frank A. Chapman, fchapman@ufl.edu, Department of Fisheries and Aquatic Sciences, University of Florida; and Joel P. Van Eenennaam, jpvaneenennaam@ucdavis.edu, Department of Animal Science, University of California-Davis.
The Institute of Food and Agricultural Sciences (IFAS) is an Equal Opportunity Institution authorized to provide research, educational information and other services only to individuals and institutions that function with non-discrimination with respect to race, creed, color, religion, age, disability, sex, sexual orientation, marital status, national origin, political opinions or affiliations. For more information on obtaining other extension publications, contact your county Cooperative Extension service.
U.S. Department of Agriculture, Cooperative Extension Service, University of Florida, IFAS, Florida A. & M. University Cooperative Extension Program, and Boards of County Commissioners Cooperating. Larry Arrington, Dean.

