The onset of sexual maturation in Atlantic salmon Salmo salar is a flexible process, the timing of which can be influenced by numerous environmental factors including photoperiod, water temperature, feed intake, nutrition, lipid reserves, growth rate and stock genetics.
Early maturing males are known to occur in wild populations, and in the salmon farming industry precocious males (‘jacks’ or ‘grilse’) are highly undesirable due to relatively poor growth performance and feed conversion efficiency, as well as reduced product quality.
Because grilsing can result in serious economic loss to Atlantic salmon farmers, strategies for reducing early maturation have been developed and include photoperiod control, selective breeding and triploidy.
The concept of raising Atlantic salmon to market size in land-based, closed-containment water recirculation facilities, versus grow-out in coastal net-pens, is gaining considerable attention from aquaculture researchers.
As raising salmon to market size in water recirculation aquaculture systems (RAS) is presently a frontier in aquaculture, there have been issues encountered in research grow-out trials, including a high prevalence of precociously maturing male salmon in the water recirculation environment.
On-site research has demonstrated that, under a 24 h photoperiod, up to 80% of male salmon are sexually mature prior to harvest at 4 kg average final weight. Published research combined with anecdotal evidence provided to us by industry personnel, however, suggested that rearing salmon at a reduced photoperiod (i.e. 18 h light/6 h dark) during their first-year post hatch, followed by a constant photoperiod until harvest, will decrease the level of precocity observed during grow-out.
This novel photoperiod approach was investigated by raising Atlantic salmon from first feeding up to 12 months post hatch under either constant (i.e. 24 h) or reduced (18 h:6 h) photoperiod.
The only break in this treatment regimen was to provide, beginning at approximately 40 g average weight, a 6-week S0 ‘winter’ (i.e. 12 h:12 h) photoperiod, as per industry recommendations, in order to induce smoltification in both groups, after which the two groups were returned to their original photoperiod treatment.
After the first year of rearing, all fish in the 24 h group were adipose fin-clipped for later identification, and then all fish from both groups were transferred to a semi-commercial scale RAS and raised to 24 months post hatch, at which point they were depurated and harvested as food fish.
The only fish removed from the semi-commercial scale RAS prior to the final harvest were (1) occasional culls due to external Saprolegnia spp. infections and (2) all visibly mature males (grilse) at 19 months post hatch. During this latter grilse harvest, fin clips were noted to determine the photoperiod treatment regime for each fish removed.
Aside from the photoperiod treatment, all other conditions remained the same between the two groups, including feed and feeding rates, water quality and stocking densities.
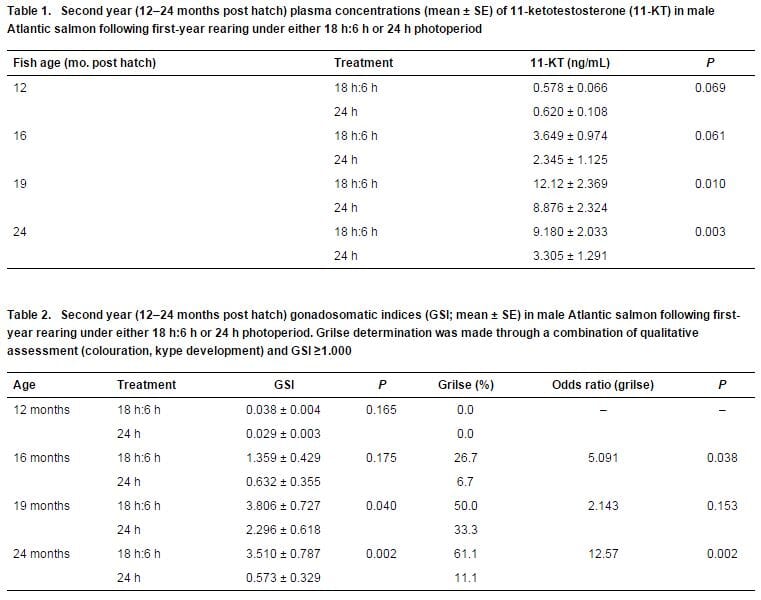
To assess maturation, samples of 30 males from each treatment group were collected at 12 months (just prior to fish transfer to the grow-out RAS), 16 months, and 19 months (just prior to the grilse harvest) post hatch in fish age, at which times data were collected for (i) gonadosomatic indices (GSI) and (ii) blood plasma 11-ketotestosterone (11-KT) concentrations. 11-KT is the major androgen produced by the testes in salmonids and is considered an indicator for sexual maturation.
An additional final sample was collected at 24 months post hatch, prior to the final harvest, although due to the removal of grilse at 19 months post hatch the majority of fish in the population during the final sampling was female, and hence only 18 males per treatment group were able to be identified and sampled at that point.
The overall finding of this study is that the reduced first-year photoperiod did not impart the expected reduction of male sexual precocity in the second year of grow-out; rather, the reduced photoperiod appeared to be associated with a significantly increased level of maturation via the majority of assessments carried out at 16-, 19- and 24 months post hatch in fish age.
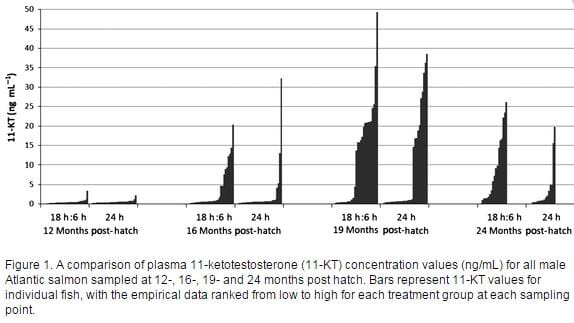
Although mean plasma 11-KT was initially slightly higher at 12 months post hatch in the 24 h photoperiod group, all subsequent assessments indicated higher mean 11-KT concentrations in the 18 h:6 h group, with significantly higher levels at 19- and 24 months post hatch in this group (Table 1; Figure 1).
Likewise, mean GSI values were significantly higher in the 18 h:6 h treatment group at 19- and 24 months post hatch (Table 2).
Although grilse were observed in both treatment groups during the 16-, 19- and 24 month sampling events, the odds of grilse being from the 18 h:6 h treatment group were significantly higher at 16- and 24 months post hatch.
Males sampled at the 19-month event, just prior to the overall population grilse harvest, were 50.0% grilse in the 18 h:6 h group and 33.3% grilse in the 24 h group.
Although this difference among sampled fish was not statistically significant, full population data collected subsequently can be compared; these grilse harvest data indicated that among all grilse removed (17.0% of the total salmon population; 34.0% of the entire male population), 60.2% were from the 18 h:6 h treatment group and 39.8% were from the 24 h group.
Grilse harvest population data also indicated that, at 19 months post hatch, 40.9% of all males (20.4% of the total population) in 18 h:6 h group were sexually mature, while 27.0% of all males (13.5% of the total population) in the 24 h group were mature.
April 2015