The biological filter, or biofilter, is a key component in the filtration portion of a recirculating aquaculture system (RAS). The biofilter houses the nitrifying bacteria and is the primary site where biological nitrification occurs. Nitrifying bacteria process dissolved nitrogenous waste products excreted by the aquatic organisms being cultured.
All cultured organisms, vertebrates or invertebrates, finfish or shellfish, produce waste as a result of the nutrition they receive. Finfish excrete ammonia (NH3), mostly from their gills, and it dissolves in the water in which the fish must live. This waste product is toxic to the fish and is an environmental stressor that causes reduced appetite, reduced growth rate, and death at high concentrations.
Fortunately, naturally occurring bacteria oxidise the ammonia, use it to grow, and convert it to nitrite (NO2-). This is an aerobic process that needs oxygen to occur. The bacteria that convert ammonia to nitrite are known collectively by their genus name Nitrosomonas. Like ammonia, the nitrite produced by the Nitrosomonas bacteria is toxic to aquatic organisms and must be oxidised further to a less toxic form of nitrogen. This is accomplished by another naturally occurring genus of bacteria referred to as Nitrobacter. These bacteria metabolize and oxidise the nitrite (NO2-) produced by Nitrosomonas and convert it to nitrate (NO3). Oxygen is also consumed in the creation of nitrate. Nitrate is the end product of the conversion of ammonia and nitrite and is non-toxic at the levels usually found in an RAS (>200 mg/L). Further information can be found in SRAC Publication No. 451, Recirculating Aquaculture Tank Production Systems: An Overview of Critical Considerations.
The reaction sequence accomplished by the nitrifying bacteria can be simplified as follows:

An excellent, detailed discussion of nitrifying bacteria and the nitrification process can be found in Hagopian and Riley (1998).

What does starting a biofilter involve?
Starting a biofilter involves managing and controlling the seeding of nitrifying bacteria cells in a biological filter. A biological filter consists of non-corroding material such as plastic, fiberglass, ceramic or rock that has large amounts of surface area nitrifying bacteria cells can colonise. To make biofilters more compact, material that has a large surface area per unit volume is usually chosen. This unit of measure is usually referred to as the specific surface area (SSA) of the biofilter media. Simply stated, the more surface area available, the more bacteria cells can be grown and the greater the nitrification capacity, which means that higher feed rates can be achieved. A biofilter with a higher SSA will be more compact than one with a lower SSA.
Keep in mind, however, that some biofilter media with a higher SSA can become clogged with bacteria. Thus, there must be a balance between a high SSA and an operationally reliable biofilter media. Nitrifying bacteria cells grow on all surfaces of the biological filter media and, in fact, on all wet surfaces of the system, such as the insides of pipes, tank walls, etc.
Bacteria follow a continual cycle of growing and multiplying, maturing and dying, sloughing off of the media, and being replaced by new cells. A biofilter is started by adding bacteria to the system, which can be done in several ways. Nitrifying bacteria can be introduced with water or bits of biofilter media from an already operating system, with pond sediment or barnyard soil, or with small numbers of “starter” animals. These animals will have to survive the rigours of elevated ammonia and nitrite concentrations while bacteria cells reproduce and colonise the biofilter media.
Whatever method is used to add bacteria to a system, there is a danger of introducing pathogens as well. The choice of a method should be evaluated as part of the overall facility management and biosecurity plan.
One strategy for starting a biofilter, sometimes called the cold start method, involves stocking the cultured species without having an activated biofilter. Operators must be prepared to deal with the resulting rapid increase in ammonia and nitrite concentrations and to reduce them to tolerable levels through water exchanges. Feed rates must be reduced or feeding must be suspended until biofilter activation occurs. The cold start method has the advantage of using the bacteria that entered the system with the first animals introduced, bacteria that must have been well-suited to the conditions from which those animals came.
This passive biofilter activation, however, can be a slow and stressful process for the animals and the system operator. A more desirable method is to develop the nitrifying bacteria within the biofilter before stocking the species to be cultured. The advantages of “seeding” nitrifying bacteria are:
- Reducing stress on the newly introduced stock
- Shortening the growing cycle with higher feed rates from the first day of stocking (which is important to the economics of an RAS)
- Creating better water quality, which improves health, growth rates, and survival.
Steps in starting a biofilter
To start a biofilter you must have a source of nitrifying bacteria and create conditions that will encourage their growth. The following procedure assumes that animals are not yet in the system. Adding nutrients to support the growth of bacterial cells can create concentrations of ammonia and nitrite that can be lethal to your cultured species.
1. Prepare the water chemistry of the system before introducing either nitrifying bacteria or animal stock
The system should be operating and passing water through the biofilter. If the culture tank is not yet ready for use, it is possible to recirculate water only through the biofilter unit. This situation may arise when starting a newly built facility. System water should be free of residual chlorine that may have been used for disinfection or pathogen control. System water should be warmed or cooled to near the temperature at which the system will be operated when stocked; pH, salinity, alkalinity and hardness should match the requirements of the incoming stock.
2. Provide alkalinity, a carbon source
Carbon dioxide dissolved in the system water is a source of carbon for the developing bacteria cells, but carbonate (CO3-2) and bicarbonate (HCO3-) ions are also carbon sources and are more easily added and controlled.
Add sodium bicarbonate (NaHCO3), or common baking soda, to increase alkalinity. Baking soda is inexpensive and safe to use. Increase system alkalinity to about 150 mg/L initially. Alkalinity at this level will support the growth of Nitrosomonas bacteria, but the authors have had better success in establishing Nitrobacter bacteria at a higher alkalinity of about 200 to 250 mg/L. When Nitrobacter does become established, alkalinity can be allowed to decline to operational levels. The alkalinity of your water source will affect the quantity of sodium bicarbonate needed to raise the system alkalinity to suitable levels. As a rule, to raise the alkalinity by 10 mg/L, add 53 grams of sodium bicarbonate for every 3,785 litres (0.117 pounds or 1.86 ounces per 1,000 gallons) of system water capacity. Refer to Figure 2 in SRAC Publication No. 452, Recirculating Aquaculture Tank Production Systems: Management of Recirculating Systems, for a complete graphic summary of pH and alkalinity management.
3. Adjust pH if necessary
With alkalinity at the level suggested above, pH is usually not a problem. A range of 6.8 to 7.2 is best. The lower healthy limit of pH for nitrifying bacteria, about 6.8, is usually not reached unless a high concentration of carbon dioxide is present. During the start-up of a new system, this is unlikely unless the source water is high in carbon dioxide. Circulation and aeration or degassing of the water in the system will reduce CO2 concentration.
4. Provide ammonia and nitrite
Add ammonium hydroxide, or unscented household ammonia used for cleaning, which is an aqueous solution of ammonia. Ammonium chloride and ammonium nitrite also are commonly used. Add sufficient ammonium hydroxide to raise the total ammonia level to between 3 and 5 mg/L. This should allow for good detection by most testing methods. Generally, 60 ml of clear household ammonia (10 percent aqueous ammonia) will raise the ammonia concentration of 3,785 litres (¼ cup per 1,000 gallons) of system water by about 1.6 mg/L. Adjustments will be necessary if you are using a different concentration of aqueous ammonia. Start with a minimum amount, allow enough time for thorough mixing through the system, and then test for the ammonia concentration. If more is needed to increase the concentration in the system to between 3 and 5 mg/L, add more incrementally.
5. Introduce nitrifying bacteria
Introducing bacteria may accelerate the acclimation process. If using commercial preparations of bacteria, follow the manufacturer’s recommendations during the previous steps and in adding the bacteria.
Apply the suggested nutrients at the rates and schedules directed by the manufacturer. Directions may suggest that bacteria be added in several portions. The most effective way to introduce bacteria is to move biomedia elements from an acclimated system operating under similar culture conditions. If introducing already colonised biomedia elements or other forms of bacteria, add them at this time.
6. Begin monitoring water quality parameters
Water quality parameters should be monitored regularly during the start-up of a biofilter. Ammonia, nitrite, pH, temperature and alkalinity are the primary water quality parameters to test. Reliable, accurate and repeatable methods for measuring ammonia and nitrite concentrations in the system should be established.
Testing can be done with test kits and colour comparators, colorimeters, or spectrophotometers. Operators should be able to compare changes in water quality from one day to the next with confidence that the test results are representative of changing water quality conditions that the biofilter is causing.
Water samples should be collected from the culture tank, since that is where the animals will be. Samples should be collected from the same place in the tank and analysed promptly. They should be taken at the same time each day, and persons conducting tests should follow the same procedures, using the same accurate measuring equipment.
Standardisation, routine, and attention to detail ensure that you will have accurate information for making operational decisions. It is helpful to graph both ammonia and nitrite concentrations, adding each day’s readings over time. This gives you a visual concept of biofilter development. Figure 1 shows the characteristic curves associated with biofilter start-up.
While the numbers may vary among different cycles, systems and biofilters, the shape and relationship of the curves should be about the same. Initially, ammonia concentration increases to a high point and then declines. After a lag period, nitrite concentration begins to increase to a point and then declines. It is also important to note that this example start-up occurred over a short period of time because of the warm temperature under which this particular trial was conducted — 28 °C (about 83 °F). Compare this graph to Figure 3 in SRAC Publication No. 452, Recirculating Aquaculture Tank Production Systems: Management of Recirculating Systems.
7. Be alert for the decline in nitrite concentration
In Figure 1, note the trend for declining nitrite concentration. The top of the nitrite curve represents the point at which nitrobacter bacteria have developed in numbers sufficient to consume more nitrite than is being produced or added to the system; thus, the nitrite concentration decreases. This trend will continue until nitrite concentration levels off. At that point, if nitrite and ammonia levels are acceptable for the species being cultured, stocking can occur. If stock is present in the system, feeding can begin at low levels, and normal production operations can commence.
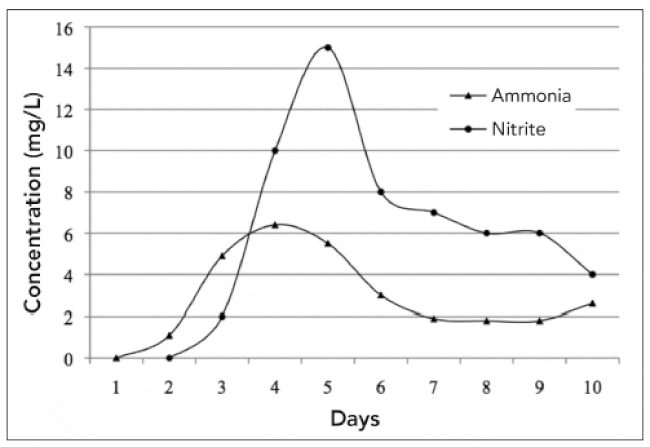
© DeLong, unpublished data, North Carolina Fish Barn, July 1998
With some growout cycles in an RAS it is sometimes unnecessary to restart the biofilter. If a system is being transitioned from one production cycle to the next, the system can be kept operating and the biofilter kept active between cycles, stocking the next cohort immediately after harvesting or transferring the previous one.
In the interest of disease control, which is critical in commercial aquaculture production, it is often necessary to disinfect the system upon completion of a growth cycle. Disinfection is normally accomplished by flooding the tanks, filtration components, and pipes of the entire system with a solution of water and bleach (chlorine) or other suitable disinfectant and allowing it to circulate, followed by draining and drying of the system.
This process kills not only pathogens in the system but also the nitrifying bacteria in the biofilter, which must then be started anew for the next growth cycle. If a biofilter is kept alive and functioning between production cycles, some bacterial cells will be lost because the amount of ammonia and nitrite available to sustain the bacteria population has decreased. Some cells may slow down their processes, and the biofilter’s overall nitrification capacity will decrease slowly.
Nitrifying bacteria activity will increase again as soon as animals are fed and waste production increases. However, feed rates should be increased slowly, as it will take some time for the bacterial biomass to return to pre-harvest levels. If there will be several days or weeks between cycles, doses of ammonia can be added to the system to keep the bacteria alive and functioning.
Using bacterial preparations
A number of prepared mixes of cultured nitrifying bacteria are available from suppliers, for both freshwater and marine applications. These are available as dry powders or concentrated liquid suspensions. They may arrive as a chilled culture along with the inorganic chemicals needed for their initial nutrition as they become established. Although they are not essential, these bacterial preparations can shorten the time that it takes to establish the biofilter.
When using these preparations, follow the manufacturer’s recommendations. Some operators report, and the authors have experienced, that bacterial cultures are essential for starting biofilters for brackish water or marine culture conditions (>15 ppt salinity). There are species of nitrifying bacteria in nature that have adapted to any salinity that might be encountered, and some species have broader or narrower ranges in which they thrive.
Tips and tricks
Temperature can be raised slightly to increase the rate of reaction of biological and biochemical processes, thus speeding the development of a nitrifying bacteria population. Increase the temperature no more than 2 to 3 °C (3.6 to 5.4 °F) above the anticipated culture temperature to avoid a major die-off of bacteria when the temperature is reduced to operational levels. The total volume of a system can be reduced by operating the culture tanks with a lower water level during the start-up of the biofilter. With a smaller volume of water, temperature can be changed more quickly and the quantity of nutrients required for dosing the biofilter will be reduced, which makes the process more cost-effective.
It can be difficult to start a biofilter containing brand new media, possibly because the media may still have a slick, shiny surface to which it is more difficult for bacteria cells to attach. Once the first growth of bacteria is established, however, subsequent cycles usually start more quickly.
Some polystyrene bead material has shown initial resistance to bacterial colonisation. It has been suggested that additives such as flame retardants on the surface of the beads may inhibit the initial growth of bacteria. The resistance of the bead material to colonisation by nitrifying bacteria appears to be short-lived, however, and most operators work through the problem relatively quickly.
With moving bed or mixed bed biofilters, bacteria may be slow to colonise because of excessive agitation or aeration of the media bed. This is essentially a mechanical problem caused by the abrasion of the surface of the biofilter elements, continually scraping off bacteria cells that are trying to adhere. Reducing the physical agitation of the media bed by reducing aeration and/or water flow through the bed can help.
As more bacteria cells become established on the media surfaces, as evidenced by daily decreases in ammonia and nitrite concentration, aeration and agitation can be gradually increased.
Adopt the method that works best for your operation and your systems. If you are learning to start and operate systems, keep records and notes of your experiences so that you can determine what works or doesn’t work for your situation.




