Introduction
Streptococcus iniae, a Gram-positive
bacterium, causes substantial mortality
in tilapia, Oreochromis spp., especially
among fish cultured in recirculating or
intensive flow-through systems. Worldwide
annual economic loss as a result of S. iniae-associated
mortality in tilapia has been
estimated to be about US $100 million.1
Consequently, MSD Animal Health is
seeking US approval of Aquaflor, a feed
premix containing the broad-spectrum
antibacterial agent florfenicol (50% w/w;
Figure 1A), for treatment of S. iniae in
tilapia. Use of trade or product names
does not imply endorsement by the
US government.
Aquaflor was recently approved in the US
at a dose rate of 10 mg/kg bodyweight per
day (BW/day) administered in feed for 10
days to control mortality due to enteric
septicemia in catfish (2005) and coldwater
disease and furunculosis in trout (2007); it
was conditionally approved (Aquaflor-CA1)
to control mortality due to columnaris
disease in catfish (2007).
Globally, Aquaflor, which is also marketed
as Aquafen and Florocol in some regions,
is registered for use in more than 20
countries including Norway (1993); Chile
(1995); Canada (1997); the United Kingdom
(1999); Ecuador and Venezuela (2005);
Colombia (2006); and Brazil, Costa Rica,
Vietnam and China (2007) to control
various susceptible pathogens in a variety
of commercially important freshwater
and marine species.
Chemical Structures of Florfenicol (1A) and Florfenicol Amine (1B)**
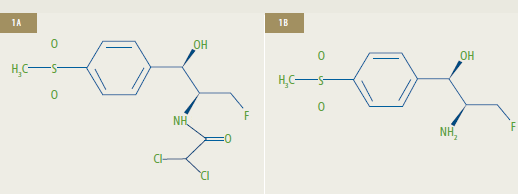
**1A: Florfenicol [R-(R*,S*)-2,2-dichloro-N-[1-fluoromethyl-2-hydroxy-2-(4-methylsulfonylphenyl)]
ethyl acetamide] is the active ingredient of Aquaflor.
1B: Florfenicol amine [R*,S*0]-?-(1-amino-2-fluoroethyl)-4-(methylsulfonyl)-benzenemethanol])
is the marker residue of florfenicol.
We evaluated the depletion of florfenicol amine (FFA) a marker of florfenicol (FFC) residue from tilapia fillet and the decline of FFC following FFC-medicated feed administration at a nominal dose rate of 20 mg/kg BW/day to fish reared in a recirculating aquaculture system (RAS). The objective of the study was to develop the marker residue depletion data needed to allow FFC administration at a proposed maximum dose of 15 mg/kg BW/day for 10 consecutive days.
Florfenicol Depletion
FFC distribution, metabolism and
depletion following dosing at 10 mg/kg
BW/day has been well characterized in a
variety of fish. FFC was similarly
distributed in freshwater- or seawater-acclimated
tilapia with the maximum
concentration occurring 2 to 24 hours
post-dosing, depending on the tissue.
In Atlantic salmon, FFA (Figure 1B) was
identified as the primary metabolite of FFC
in muscle. Muscle (skin-on fillet), by
regulation, is considered the edible
tissue of most fish. FFA was subsequently
selected as the marker residue of FFC
administration because it is the primary
FFC metabolite, and other lesser
metabolites (and FFC) are converted to
FFA through acid hydrolysis.
Monitoring total FFA concentration (FFA +
acid-hydrolyzed FFC and metabolites) in
the target tissue thus provides a conservative
estimate of FFC residues and enables
calculation of a conservative withdrawal
period. Although FFC metabolism data
were not available for tilapia, FFA was assumed
to be the marker residue since it is
the marker residue in cattle, swine, sheep,
poultry, catfish, salmon and trout.
Data from residue depletion studies are
used to calculate a withdrawal period for a
drug, which is the time required for the animal
to deplete the drug residue to a level
that is considered safe for human consumption.
Regulatory agencies estimate
the safe concentration or maximum residue
level (MRL) by combining an acceptable
daily intake (ADI) level (from toxicology
data) with a standard human mass estimate
and a consumption factor (an estimate
based on the mass of residue-bearing
tissue consumed).
For FFC, the 10 ?g/kg ADI is multiplied
by a standard human weight (60 kg), then
divided by a consumption factor (in fish,
a standard mass [300 g] of skin-on fillet
[muscle] is used), resulting in a tolerance
of 2 ?g/g; the European Agency for the
Evaluation of Medical Products and the
US Food and Drug Administration have
applied an additional safety factor and
established the MRL for Europe and the
US at 1 ?g/g.
Materials and Methods
Commercial tilapia culture is principally
focused on the rearing of phenotypic males
produced by the administration of feed
containing 17?-methyltestosterone (MT)
to tilapia fry. Tilapia used in the study
were a mix of MT gender-reversed females
(phenotypic males) and genetic males of
the two most commonly cultured tilapia
strains, pure Nile tilapia (O. niloticus x
O. niloticus) and hybrid tilapia (O. niloticus
x O. aureus).
Aquaflor-medicated premix was used
to prepare the medicated feeds. The
feeds used were assayed for FFC content
by high-performance liquid chromatography15
(HPLC) before and after dosing
(Table 1). The non-medicated feed was
analyzed to determine proximate nutrient
content as well as inorganic or organic
contaminants (Eurofins Scientific Inc., Des
Moines, Iowa, USA). Non-medicated feed
and feed medicated with Aquaflor (2.667 g
FFC/kg) for the residue depletion study
(extruded 4.8 mm floating pellets) were
prepared according to standard procedures
at Delta Western Research Center
(Indianola, Mississippi, USA). Aquaflor
premix was added to the medicated feed
during mixing, prior to extrusion.
Nile and hybrid tilapia (mean weight
= 447 56 g) were obtained from a
commercial farm. Tilapia were held in a commercial RAS (Aquatic Eco-Systems
Fish Farm II) consisting of twin ~1,900 L
(500 gal.) polyethylene tanks, mechanical
filters (clarifier and suspended solids filter)
and a biological filter; there was ~3,350 L
(885 gal.) total system water.
The RAS biofilter was inoculated with
commercial biofilter bacterial inoculum
and allowed to operate for ~6.5 months
with fish present before FFC administration.
These fish were removed, and the tilapia
used for testing were stocked into the
RAS 38 days before FFC administration.
Temperature was maintained at 27 C to
27.6 C (80.6 F to 81.7 F). Waste solids
were removed once daily ~1 hour before
feeding, and concurrent with solids
removal, a portion of the RAS water was
removed (acclimation 6-11%; dosing
and post-dosing 5-8%) and replaced with temperature-adjusted well water
(at ~22 C/71.6 F). Water chemistry
(temperature, dissolved oxygen, pH,
total ammonia, nitrite and nitrate) was
determined once daily prior to tank
cleaning. Water hardness and alkalinity
were determined weekly. Alkalinity was
maintained at >150 mg/L as CaCO3 by
occasional addition of sodium bicarbonate.
A single water sample was analyzed for
metals and volatile and semi-volatile
organics (Davy Laboratories, La Crosse,
Wisconsin, USA). No contaminants at
levels of concern were identified.
Non-medicated feed was offered at a rate
of 0.25%-1% BW/day during the 38-day
acclimation period; the feed rate was 0.75%
BW/day for the last 11 acclimation days and
remained constant through the remainder
of the study, including the dosing and post-dosing periods. Three equal feed
portions were offered each day with ~4
hours between each feed administration.
Daily feed consumption was estimated
during the dosing period.
Mean Florfenicol (FFC) Concentration* in Feed
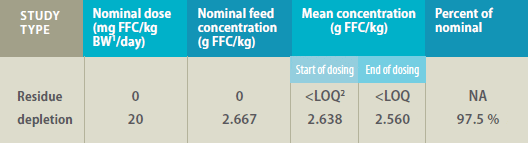
1 Bodyweight
2 LOQ = limit of quantitation (0.0002 g/kg)
*Determined by high-performance liquid chromatography in feed samples collected at the
start and end of dosing for residue depletion studies
Five fish from each tank were sampled
4 days prior to dosing to obtain control
fillet tissue. Fish were indiscriminately
removed and sacrificed; the fish were then
scaled and skin-on fillets were collected,
individually bagged and stored at <-70 C
(-94 F).
Tilapia (n = 209) were offered feed
medicated with Aquaflor at 0.75% BW/day
(nominal dose = 20 mg FFC/kg BW/day)
for 10 consecutive days. The estimated
delivered dose was calculated from the
estimated feed mass consumed, feed FFC
concentration and the total fish mass at
terminal sampling. Twenty fish (10 per
tank) were indiscriminately removed from
the RAS tanks on days 0.04, 0.5, 1, 1.5, 2, 3,
4, 5 and 10 post-dosing, and skin-on fillets
were collected as previously described.
Water samples for FFC analysis were
collected: (1) concurrent with the control
fillet collection, (2) prior to tank cleaning
during the dosing period (~1 hour prior to
the first daily feeding), (3) just prior to the
second and third daily feeding intervals, (4) 4 hours after the third daily feed
interval and (5) concurrent with tissue
collection during the post-dosing period,
except the 0.04-day post-dosing fillet
collection. At each collection interval,
one water sample (~50 mL) was taken
from the RAS clarifier and from the RAS
suspended solids filter. Each sample
was hand-mixed and syringe-filtered
(Durapore [PVDF, 0.45 ?m] membrane,
Millipore, Billerica, Massachusetts, USA)
in ~2-mL aliquots into HPLC vials then
stored at ?-20 C (?-4 F) until analyzed.
FFA concentrations were determined using
a method validated for FFA in tilapia fillet
tissue at MPI Research, Inc. (State College,
Pennsylvania, USA). The method involved
converting all FFC residues to FFA by
acid-catalyzed hydrolysis. Fillet tissue was
hydrolyzed by adding 6N hydrochloric acid
then held for approximately 2 hours at
95 C to 100 C (203 F to 212 F). The
tissue-hydrolysate was extracted with ethyl
acetate and centrifuged. The aqueous
hydrolysate was retained and adjusted to
pH 12.5 or greater with 30% (w/w) sodium
hydroxide solution. The pH-adjusted
solution was adsorbed from 45 to 60
minutes onto a Varian Chem Elut CE120
sorbent column (Varian, Inc., Palo Alto,
California, USA) then eluted with methylene
chloride. The methylene chloride
eluates were evaporated to dryness, dissolved in 10 mM potassium phosphate
buffer (pH 4.0, 1% [v/v] acetonitrile),
filtered (0.2 ?m) and then analyzed by HPLC
using UV detection at 220 nm. The method
quantitation limit (LOQ) was 0.05 ?g/g.
FFC concentration was determined in water
samples using a validated determinative
procedure capable of quantifying FFC from
10 to 5,000 ng/mL and up to 20,000 ng/mL
after dilution. FFC concentration was
determined by ultra-pressure liquid
chromatography with mass spectrometric
detection on an atmospheric pressure
ionization interface. Water samples
were fortified with FFC-d4 as an internal
standard and analyzed directly. Ionic
transitions of 356 to 185 m/z and 360 to
189 m/z were monitored for FFC and the
internal standard, respectively. The method
LOQ was 10 ng/mL.
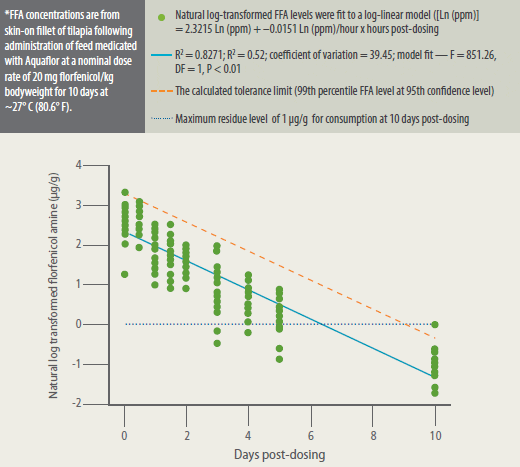
The residue depletion profile of FFA in
skin-on fillet of tilapia following withdrawal
from the medicated diet was estimated by
log-linear regression.16 The fitted loglinear
regression model ([Ln (ppm)] =
2.3215 Ln (ppm) + 0.0151 Ln (ppm)/hour
x hours post-dosing) R2 was 0.8271. The
withdrawal period was defined as the time
when the tolerance limit of the residue
concentration was at or below the 1 ?g/g
MRL. The tolerance limit was set as the 99th
percentile of the potential residue level at
95% confidence.16 The withdrawal period
was, therefore, equivalent to the time at which the tolerance limit was less than 1
?g/g. Analyses were considered significant
if P < 0.05.
Results
The mean minimum daily delivered doses
were 19.4 mg/kg BW/day for tilapia in tank
1 (range 19.3 to 19.57) and 19.8 mg/kg
BW/day for tilapia in tank 2 (range 19.7 to
20.0) or 97% to 99% of the target dose. Fish
consumed 100% of the feed medicated
with Aquaflor that was offered during the
10-day dosing period, similar to those of
non-medicated feed during the acclimation
and post-dosing periods. FFA concentrations
in tilapia fillets are summarized in
Table 2. Representative analytical standard,
control and treated tissue chromatograms
are presented in Figure 2.
Mean FFA concentration in tilapia fillets
exceeded the MRL during the post-dosing
period until the last post-dosing fillet
collection (10 days post-dosing) when all
fillet concentrations were below the MRL.
Mean FFA concentrations steadily declined
during the post-dosing period. Fillet FFA
concentrations were similar between the
two study tanks throughout the postdosing
period. FFA level depleted rapidly
to below the MRL, and the calculated
tolerance limit was below the MRL at 10
days after withdrawal of the medicated
feed (Figure 3).
Mean Florfenicol Amine (FFA) Concentration* in Fillet Tissue Following Administration of Feed Medicated with Aquaflor
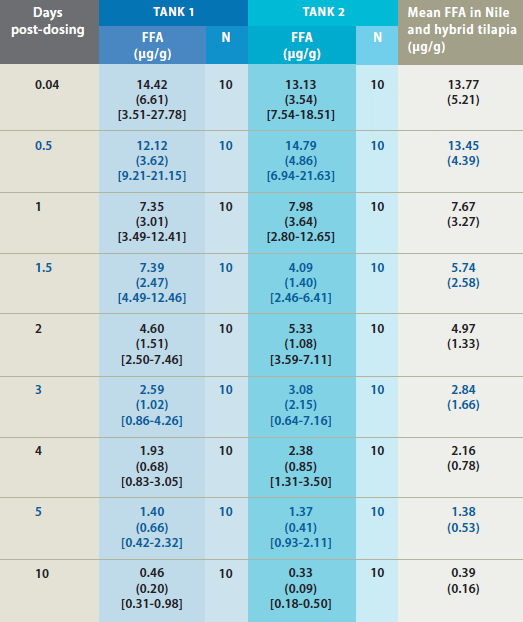
* FFA concentration in fillet tissue from Nile and hybrid tilapia following administration of feed medicated with Aquaflor as the sole ration for 10 consecutive days. Standard deviations are in parentheses and the range is in brackets. Only samples above the FFA quantitation limit of 0.05 ?g/g were included in summary calculations.
FFC levels in RAS water before dosing were
Unionized ammonia-nitrogen levels were
<0.02 mg/L NH3-N during the acclimation,
dosing and post-dosing periods. Nitratenitrogen
levels fluctuated in RAS from
between 7 to 123 mg/L with mean
concentrations of 67, 81 and 75 mg/L
during the acclimation, dosing and postdosing
periods. Nitrite-nitrogen levels
occasionally exceeded the safe upper limit
of 2.0 mg/L but only during the acclimation
period; nitrite levels were <0.9 mg/L during
the 8 days prior to dosing. Nitrite levels
steadily increased during the dosing
period until peaking on dosing day 8 at
1.76 mg/L (Figure 5). This concentration
increase was apparently not associated
with any effect on the RAS biofilter but
rather the inadvertent buildup of biofilm
material in the water supply lines to the biofilter from the RAS tanks as nitrite levels
rapidly decreased during the remainder
of the dosing period and during the postdosing
period after the water supply lines
were flushed. Nitrite levels again spiked
during the post-dosing period then
dropped after the RAS biofilter supply
lines were flushed (Figure 5).
Representative Chromatograms* of Extracts from Control and Treated Tilapia Fillet Tissue
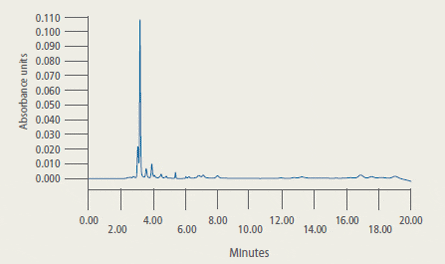
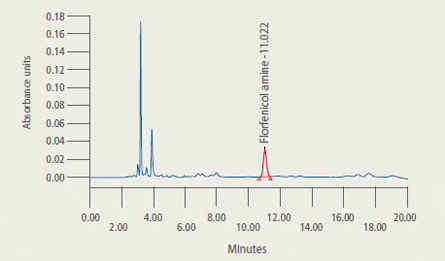
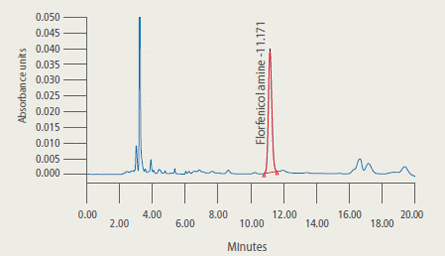
* 2A: Extract from control tilapia fillet tissue sample
2B: Extract from control tilapia fillet sample fortified with florfenicol amine (FFA) at 2 ug/g
2C: Extract (2.33 ug/g FFA found) from fillet tissue taken from a fish at 5 days post-dosing
Discussion
Fish readily consumed feed medicated
with Aquaflor. There was no reduction in
feed consumption during the dosing
periods. There do not appear to be any
palatability concerns regarding feed
medicated with Aquaflor.
FFC concentration increased in RAS water
during the dosing period then gradually
decreased during the post-dosing period.
The apparent concomitant increase in
nitrite concentration in the RAS does not
appear to correlate to RAS-water FFC
concentration. Rather, the increase in RAS
nitrite concentration was apparently due
to microbial growth in the biofilter water
supply lines restricting water flow to the
RAS biofilter, as flushing those supply lines
was followed by a rapid decline in RAS
nitrite concentration. Some antibiotics
(erythromycin, oxytetracycline) have been
found to negatively affect denitrification in
aquatic systems, albeit at levels much
higher than observed in the present study.
FFC is known to be rapidly and completely
distributed in fish4-5,7-8,10-11,18 during dosing
and to rapidly deplete from tissues6,9,17,19
after withdrawal from medication, which
are excellent traits for use in food fish to
control susceptible bacterial infections.
However, its rapid elimination means that
tissue FFC levels will rapidly decrease after
fish are withdrawn from the medicated
feed. While minor differences in study
design (e.g., feeding procedure, test
temperature, feed rates) preclude direct
comparison between FFA-residue depletion
studies conducted with tilapia fed FFCmedicated
feed while held in flow-through
systems17,19 and this study, there do
not appear to be substantial differences
between the depletion of FFA from skinon
fillet of tilapia fed FFC-medicated
feed whether fed in a RAS or in a flow-through
tank.
This rapid clearance indicates that there is
likely to be only a short post-treatment
therapeutic effect associated with Aquaflor
administration, such as when FFC levels
are at or above the bacteria MIC. Without
concomitant steps by the farmer to
reduce disease transmission (e.g., water
disinfection, improved husbandry,
vaccination), re-infection of treated fish
is possible.
As with other antibiotics, Aquaflor should
not be expected to eliminate the need for
proper husbandry practices but should
be regarded as one effective tool in the
management of disease in aquaculture.
Conclusions
FFA, a marker for FFC residue, depletes
rapidly in the skin-on fillet tissue of
tilapia following the withdrawal of feed
medicated with Aquaflor. When dosed at
19.62 mg FFC/kg BW/day for 10 days, FFA
detected in the skin-on fillet of tilapia
depleted to less than the MRL in all
samples collected 10 days after the end
of dosing. Based on these residue data and our interpretation of published regulatory
guidance, FFA should deplete from tilapia
treated with Aquaflor at 1.31 times the
maximum proposed dosage (15 mg/kg
BW/day for 10 consecutive days) to a level
safe for human consumption 11 days
after treatment.
Florfenicol Amine (FFA) Concentrations* in Fillet Tissue of Tilapia
October 2012




