
Atmospheric pressure results from the
weight of atmospheric gases pressing
down on the earths surface. The atmospheric
gases and their proportions are:
nitrogen, 78.084%; oxygen, 20.946%;
argon, 0.934%; carbon dioxide, 0.038%;
water vapor and other gases, 0.036%.
According to Daltons Law of Partial
Pressures, each gas in the atmosphere
exerts a pressure (partial pressure) in
direct proportion to its percentage composition. For example, standard atmospheric
pressure at sea level is 760 mm of
mercury (Hg), and the partial pressure of
nitrogen is 593.4 mm (760 mm x
0.78084).
Henrys Law states that each gas has a
characteristic solubility, and its molecules
will diffuse from the atmosphere into
water until the pressure in water equals the
partial pressure of the gas in the atmosphere.
When this state is reached, the
pressure of the gas in the atmosphere is at
equilibrium with its pressure in water, and
no further net exchange of its molecules
occurs between the atmosphere and water.
At this stage, the water is said to be saturated
with the gas. When the pressures of
gases in the atmosphere and in the water
are equal, the water is saturated with air.
Under certain conditions, water can
contain either a lower or higher concentration
of one or more gases than it
should at equilibrium. When water is
undersaturated with a gas, that gas enters
the water from the atmosphere, and an
equilibrium state is attained. Likewise, if
water has more of a gas than it should a
state called supersaturation the gas will
diffuse from water to the atmosphere
until equilibrium is reached.
Such diffusion does not occur rapidly
in still water. Still water can remain undersaturated
or supersaturated for several
hours or days under certain conditions.
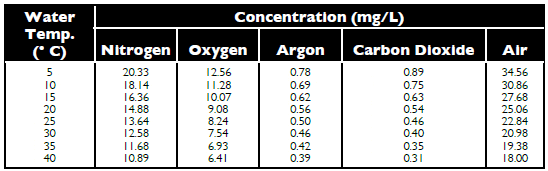
Saturation Concentration
The saturation concentrations of
gases and air vary with water temperature
(Table 1). A sudden rise in temperature
would result in temporary gas supersaturation,
while a drop in temperature would
cause temporary gas undersaturation.
Concentrations of air and individual
gases in water sometimes are given as
percentage saturation. For example, saturation
with dissolved air at 20 C is 25.06
mg/L (Table 1), but if water at this temperature
contains 30.17 mg/L air, its percentage
saturation with air is 120.4%
[(30.17/25.06) x 100]. Obviously, at
equilibrium, the percentage saturation of
a gas (or air) in water is 100%.
Supersaturation
There are several reasons why water
becomes supersaturated with air. Natural
warming of water in a pond or heating of
water in hatcheries are common causes. Air
leaks on the suction side of pumps or an
improper submergence depth of pump
intakes can cause gas supersaturation. Highly
efficient submerged aerators have also been
reported to cause gas supersaturation.
One of the best-known causes of gas
supersaturation is entrainment of air bubbles when water falls over spillways of
high dams. In cooler climates, water that
infiltrates downward into aquifers in winter
can be quite cold and contain a high
concentration of air. In warm weather,
water from wells in such aquifers tend to
be supersaturated with air in respect to
ambient air temperature.
Individual gases can be below or
above saturation, with the most common
example being dissolved oxygen. It is not
unusual for surface waters in ponds to
have dissolved-oxygen supersaturation of
200 to 300% during the afternoon
because of photosynthesis. At night, photosynthesis
stops, and respiration can
cause dissolved-oxygen concentrations to
fall to 50% or less of saturation.
Concentrations of Individual Gases and Atmospheric Air in Freshwater at Different Temperatures and 760 mm Hg.
Gas Bubble Trauma
Gases dissolve in the blood of fish,
shrimp and other aquatic animals. Suppose
animals are held in water at a certain
temperature, and their blood equilibrates
with gases in the water. Then suppose
the water is suddenly warmed, resulting
in gas supersaturation. The fish blood
also will be supersaturated with gases,
and gas bubbles can form in the blood. In
fact, anytime the blood of animals becomes supersaturated with gas, bubbles
can form.
This condition is known as gas bubble
trauma, and it can lead to stress or mortality.
Eggs may float to the surface, and larvae
and fry may exhibit hyperinflation of
the swim bladder, cranial swelling, swollen
gill lamellae and other abnormalities.
A common symptom of acute gas
bubble trauma in juvenile and adult fish is
gas bubbles in the blood that can be seen
in the surface tissues on the head, in the
mouth and in fin rays. The eyes of
affected fish also tend to protrude.
Saturation Assessment
A variable known as DeltaP is used to
assess gas supersaturation in water relative
to gas bubble trauma. The DeltaP is
defined as the difference between the
total gas pressure in water and the barometric
pressure at a given location.
The DeltaP can be calculated by measuring
the partial pressure of each gas in the
water [(percentage saturation/100 x partial
pressure in the atmosphere], summing
the partial pressures and subtracting
from the sum the barometric pressure.
Fortunately, a relatively inexpensive
instrument called a saturometer can be used to measure DeltaP directly.
Aquatic animals exposed to DeltaP values
of 25 to 75 mm Hg on a continuous basis
may exhibit some symptoms of gas bubble
trauma, and low-level mortality may
occur over an extended period of time.
Acute gas bubble trauma occurs at greater
levels of DeltaP. Symptoms will be more pronounced,
and mortalities typically are 50
to 100%.
Supersaturation of pond waters with
dissolved oxygen during afternoons is a
common occurrence. This condition usually
does not harm culture animals, for
supersaturation does not persist for long
and is often limited to surface waters.
Animals can move to greater depths,
where the combination of lower dissolved-
oxygen concentration and greater
hydrostatic pressure result in a lower DeltaP.
Nevertheless, carp reportedly had a
greater frequency of disease when percentage
saturation with dissolved oxygen
exceeded 150% (DeltaP above 225 mm Hg).
Mortality of fish and shrimp has been
reported in culture systems where dissolved-
oxygen supersaturation exceeded
300% (DeltaP above 450 mm Hg).
Management
Supersaturating gases can be removed
from water in degassing towers in which
water is passed through screens or other
media to increase exposure to the atmosphere.
In ponds, aerators that splash
water into the air can lessen afternoon
supersaturation with dissolved oxygen
caused by a high rate of photosynthesis.
Of course, managers always should guard
against gas supersaturation caused by
heating water, pumps, submerged aerators
and air entrainment.
July 2012


