Introduction
The group of Lactic acid bacteria (LAB) represents a large part of the microbiota of vertebrates and their beneficial effects on the immune system, gastrointestinal tract, and reproduction have been widely reported. Moreover, studies with germ free mice and zebrafish have shown that host microbiota can modify host nutrient metabolism and energy balance. Due to a distinct selective pressure imposed within the host’s gut habitat, the microbiota community structure is different between zebrafish and mice. We speculated that different microbiota compositions might correspond to different physiological and developmental performances; thus, in the current study we wondered what effects we could induce in the developmental profile of the host organism if we influenced the gut microbiota from birth to puberty by providing a selected LAB strain. We chose zebrafish as our model because of its short life cycle, well documented gonad developmental profile and the fact that the calcification process of the skeleton has been previously described. The LAB strain we selected was Lactobacillus rhamnosus, which is one of the main components of the commensal microflora of human intestinal tract of and is widely used as a probiotic in mammals.
Upon L. rhamnosus treatment, quantitative gene expression of a number of markers involved in stress response usually induced by captive conditions, muscle growth and development were monitored, together with growth rate of the larvae. Stress is one of the main causes affecting growth with cortisol being the primary mediator for stress response in fish; cortisol effects are mediated by the intracellular glucocorticoid receptor (GR) and GR mRNA abundance is correlated with circulating cortisol levels. Together with GR, 70-kDa heat shock protein (HSP70) has been widely used as a biomarker for stress response to captive conditions. Therefore, we used GR and HSP70 gene expression to evaluate stress status upon L. rhamnosus treatment. Eventually stress can impact the whole animal’s performance, in terms of growth and metabolism. Fish growth is positively correlated to muscle growth and is controlled by extrinsic regulators such as insulin-like growth factor-1 (IGF-1), insulin-like growth factor-2 (IGF-2) and myostatin (MSTN), which are involved in fish myogenesis and have a role in muscle growth. Moreover, gene expression of IGF-1 and IGF-2 are dependent on feeding regime. MSTN, a member of the transforming growth factor-b (TGF- b) superfamily, acts in an opposite way by inhibiting muscle cell proliferation. We tracked IGFs and MSTN gene expression to see whether L. rhamnosus administration could affect growth by positively acting on the IGF system. Indeed, growth metabolism is correlated to several other factors, some of which belong to the nuclear receptor superfamily. Peroxisome proliferator activated receptors (PPARs), retinoic acid receptors (RARs) and vitamin D receptors (VDRs), are natural receptors for many organic compounds delivered by LAB activity and modulate optimal growth and correct development. These factors affect and regulate growth and organism remodelling through processes such as lipid metabolism, energy administration and epithelial cell growth and differentiation, calcium homeostasis, growth and bone formation, morphogenesis and chondrogenesis. PPAR-a and -b are known to be involved in lipid metabolism and cell growth and to be activated by fatty acids. For example, LAB fermentation products, including short-chain fatty acids and immunoregulatory molecules as eicosanoids, are known to activate PPARs and for these reasons we used PPAR-a and -b gene expression as biomarkers to evaluate the effects of L. rhamnosus on lipid production.
VDRs mediate the effects of the most bioactive derivative of vitamin D, 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3], by initiating a cascade of molecular events leading to bone mineralization and remodelling and by modulating cell growth and differentiation. RARs mediate the biological effects of retinoids, derivatives of vitamin A, which are required for cellular growth and development. In particular, RAR-c is known to be involved in correct bone development during the early developmental stages. So in this instance, we used VDR-a and RAR-c gene expression as biomarker for backbone calcification.
Concerning sexual maturation, GnRH mainly orchestrates vertebrate puberty and gametogenesis. In the two GnRH-form zebrafish species, GnRH3 has been shown to elicit gonadotropinreleasing activity and is thought to assimilate non-redundant functions of GnRH1. To understand whether the presence of L. rhamnosus could influence gonad development and sex differentiation through the GnRH pathway, we used a GnRH3-GFP transgenic line; in parallel, the ongoing backbone calcification was determined in larvae. Zebrafish is a juvenile protogynous hermaphrodite species that, irrespective of their genetic gender, first develop ovary-like gonads. Bisexual differentiation takes place when protogynous ovaries of some specimens in a population undergo differentiation, turning first into an intermediate phase termed ‘altered ovary’ and then into testes. To determine whether L. rhamnosus could act on the onset of gonad differentiation, we timed the appearance of the first testis. The progress of gonad maturation by histological analysis and the sex ratio were registered at the end of the experiment.
Upon the presence of L. rhamnosus, expression of genes related to growth and development increased and enhanced GnRH3 expression was observed; moreover, an earlier onset of backbone calcification and gonadal differentiation were found, together with significant changes in the sex ratio of the final populations.
Results
Morphometric Results
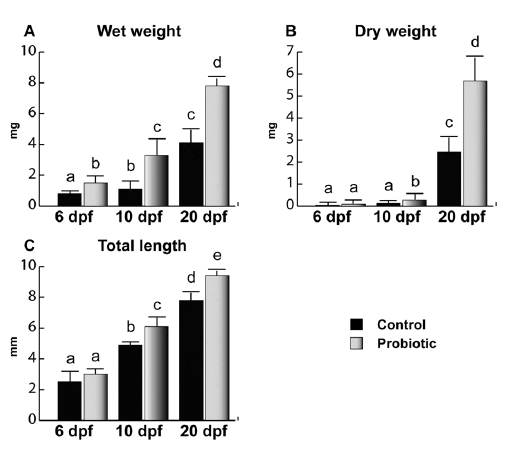
L. rhamnosus administration increased both weight (Figure 1A and B) and total length (Figure 1C). No significant difference was observed at 6 dpf for dry weight and total length, while a higher wet weight was found in probiotic fed larvae body weight and total length. At 10 dpf, a significant increase in length and body weight was observed in the probiotic fed larvae compared to control. A further increase in size was observed at 20 dpf in fingerlings treated with L. rhamnosus compared to the control.
Molecular Results
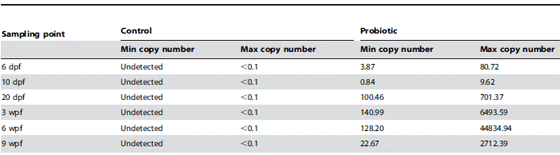
Retention of L. rhamnosus in zebrafish larvae and juveniles. Zebrafish gastrointestinal tracts retained L. rhamnosus during the entire chronic administration. In the control group, no L. rhamnosus was detected, whereas in the probiotic group, presence of L. rhamnosus was detected in both larval and juvenile stages (Table 1).
Stress Biomarkers
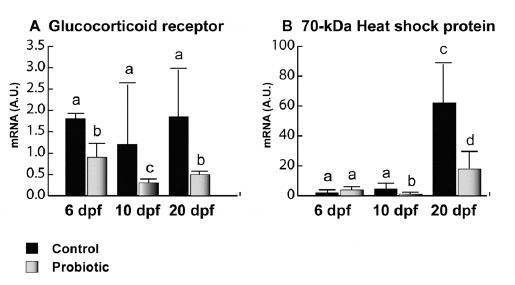
Concerning GR gene expression (Figure 2A), no significant differences were evaluated among the different developmental stages in the control; however, in the probiotic group, significantly lower gene expression levels were observed at 6, 10 and 20 dpf. Concerning HSP70 (Figure 2B), an increased gene expression trend was found in the control group from day 6 to 20 pf. In particular at 6 dpf, no significant differences were found between control and probiotic groups. At 10 and 20 dpf, significantly lower gene expression levels were registered in the probiotic treated group compared to the control.
Muscle Growth Biomarkers
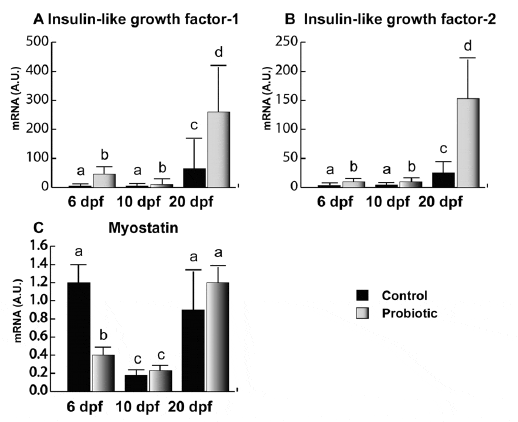
IGF-1 (Figure 3A) and IGF-2 (Figure 3B) showed very similar gene expression trends. In the control, IGFs gene expression was almost undetectable until day 20 pf. On the contrary, in probiotic group, IGF-1 and -2 gene expressions were clearly detectable as early as 6 dpf. In addition, IGFs gene expression levels were significantly higher in the probiotic group in all the developmental stages analyzed (6, 10 and 20 dpf) Concerning MSTN (Figure 3C), at 6 dpf, the probiotic group showed lower levels of gene expression with respect to the control. The lowest levels of MSTN were found at 10 dpf in both control and probiotic groups without any significant difference between the two experimental groups. No significant differences between the two experimental groups were found at 20 dpf either.
General Development Biomarkers
Analogously to what was observed for IGFs, PPAR-a gene expression (Figure 4A) in the control group was almost undetectable until 10 dpf. In contrast, at 6 and 10 dpf, the probiotic group showed higher gene expression levels. At 20 dpf, no significant differences were evaluated. Of note, gene expression levels observed in the probiotic group at 6 dpf were even significantly higher than the levels observed in the control group at day 10 pf. Also, PPAR-b (Figure 4B) was modulated by probiotic treatment; in fact, at 6, 10 and 20 dpf, higher gene expression levels were found in the probiotic group. However, PPAR-b gene expression did not seem to vary during development. Concerning VDR-a (Figure 4C), while at 6 dpf VDR-a gene expression in the control group was almost undetectable, a significantly higher level was found in the probiotic group. This level was even significantly higher than the VDR-a gene expression levels registered at 10 dpf in both control and probiotic groups. Moreover, it is noteworthy that VDR-a gene expression level in the probiotic group at 6 dpf was similar to the value registered at 20 dpf in the control group. At 20 dpf, VDR-a gene expressi on was significantly higher in the probiotic group with respect to control group.
At 6 dpf, RAR-c (Figure 4D) gene expression was almost undetectable in the control group. On the contrary, at 6 dpf in the probiotic group, a significantly higher gene expression was detected; the aforementioned level was even significantly higher than the levels at 10 dpf (in both control and probiotic groups) and 20 dpf in the control group. At 20 dpf, gene expression was significantly higher in the probiotic group with respect to control group (Figure 4D).
doi:10.1371/journal.pone.0045572.g001
GnRH3 Neuronal Development
Neuronal development was monitored using a GnRH3 transgenic zebrafish. Briefly, a 2.4 kb sequence upstream of the gonadotrophin-releasing hormone (GnRH3) decapeptide coding region including 1.3 kb of 59 flanking sequence, exon I, intron I and part of exon II was cloned and inserted into an expression vector p-EGFP1 that includes an EGFP coding sequence. Linearized construct was microinjected into the cytoplasm of zebrafish embryos at the one-cell stage. The number of pixels inside GnRH3 neurons increased in larvae fed on probiotic. In all the stages analyzed (6, 8 and 10 dpf), higher pixel numbers in GnRH3 neuron were registered in the probiotic group. We relate this finding to the elevated GnRH3 expression, which increased upon L. rhamnosus administration. From 8 to 10 dpf, larvae from control group did not show any significant change; on the contrary, at all time points analyzed, fish treated with probiotic revealed significantly higher pixel number with respect to control (Figure 5A).
Lactobacillus Rhamnosus Retention
doi:10.1371/journal.pone.0045572.g002
Backbone Calcification
Backbone centra of the probiotic-treated larvae exhibited more extensive calcification (Figure 6B) than the centra from control group’s larvae (Figure 6A). In fact, the Centra/Intracentra ratio (C/I –see materials and methods section for further description) from probiotic group was significantly higher with respect to control (2.27560.04– Figure 6D vs. 0.27660.03– Figure 6C respectively), which indicated faster backbone calcification in the probiotic treated zebrafish larvae.
Gonad Differentiation and Sex Ratio
L. rhamnosus administration significantly affected both the timing of gonad differentiation and the population sex ratio. At 3 weeks pf, in the control group, only 20% of specimens showed ovary-like gonads, while the majority of fish still showed undifferentiated gonad (Figure 7). In the probiotic group, only 10% showed undifferentiated gonads, whereas 20% of specimens had ovary-like gonads, 50% showed altered ovaries and 20% already presented testis. At 6 weeks pf (Figure 7), 80% of specimens from the control group showed ovary-like gonads and the remaining 20% of fish showed still undifferentiated gonad. In the probiotic group the 20% showed ovary-like gonads, 60% presented altered ovaries and 20% presented testis. At 9 weeks pf (Figure 7), 93% of fish from the control group were females and only 7% males. In the probiotic group, 55% were females and 45% males. No sexual behavior was observed until 10 weeks pf in both control and probiotic groups.
doi:10.1371/journal.pone.0045572.g003
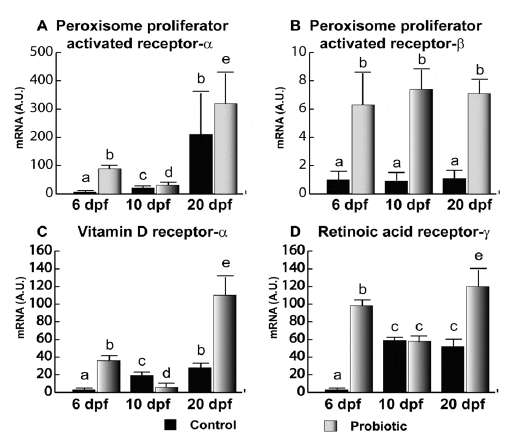
doi:10.1371/journal.pone.0045572.g004
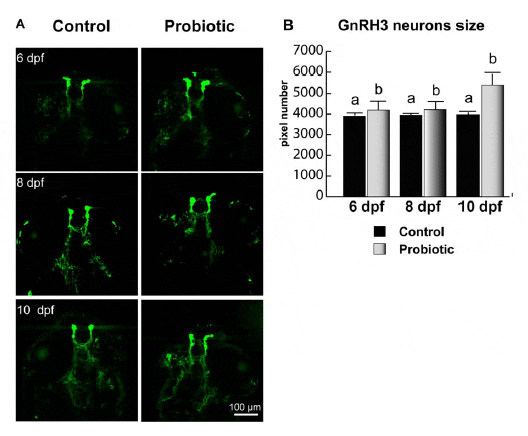
doi:10.1371/journal.pone.0045572.g005
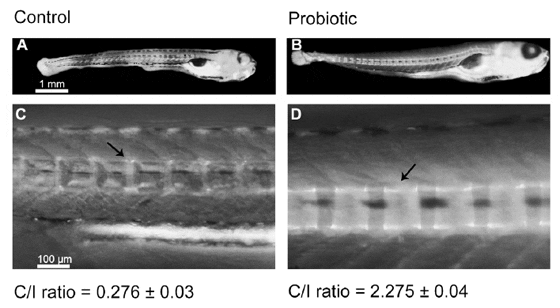
doi:10.1371/journal.pone.0045572.g006
Discussion
Modulation of host microbial community is usually associated with host physiological changes. In our study, zebrafish raised on a L. rhamnosus-supplemented diet showed faster development, with earlier onset of backbone calcification and gonad differentiation and a drastic change in the sex ratio in the final population.
Previous studies showed that beneficial microbes led an organism to more efficiently use diet-derived energy sources, leading to growth improvement. This is a likely explanation for the increase of larval size we observed in our study. Rawls and co-workers demonstrated that microbiota could modulate the expression of 212 genes, 8 of which were involved in promotion of nutrient metabolism. Analogously to what we observed in our previous work, in the present study, L. rhamnosus treatment influenced the gene expression level of the entire set of genes analyzed in a very similar fashion to what observes in the false percula clownfish, which make us infer a conserved physiological response to L. rhamnosus treatment. However here we find that L. rhamnosus can stimulate an organism developmental profile. It was not surprising that the lower physiological and cellular stress levels registered (GR and HSP70) coincided with an increase in growth. At the same time, growth-related factors (IGF-1 and IGF- 1I) showed higher levels in treated fish, while MSTN levels were significantly reduced. We hypothesized that because glucocorticoids glucocorticoids are able to inhibit IGF-1 and to increase MSTN gene expression in muscle cells, lower levels of GR gene expression yielded higher growth through IGF-signaling pathways.
Vitamin D and retinoic acid are essential molecules for morphogenesis and chondrogenesis. IGFs and vitamin D play the role as main determinants of backbone calcification and bone mass accretion, the former controlling muscle and skeletal cell growth and division, the latter stimulating calcium absorption and retention. We relate the faster calcification observed in the treated larvae with the upregulation of IGF-I and –II levels and with a plausible increased availability of LAB metabolites, as the gene expression levels of these markers revealed.
Several nuclear receptors mediate the positive action of free fatty acids and vitamins, which are compounds that are naturally delivered by LAB. PPAR-a and b, RAR-c and VDR-a were all increased by L. rhamnosus. Lactobacillus fermentation produces short chain fatty acids, which are known to activate PPARs; in agreement with previous data on mice the higher PPARs gene expression recorded could be attributed to the supplied probiotic activity in vivo. Furthermore, it is known that PPAR-a and b are involved in skeletal development, lipid metabolism and cell proliferation. Here we speculate a positive correlation between these systems and the accelerated development registered. Our observations agree with previous studies that report probiotics affecting development, specifically acting on bone accretion. We hypothesize that L. rhamnosus supplementation permits the recipient to develop faster in the following manner: 1) through the delivery of molecules such as fatty acids and vitamins as supported by the PPARs, VDR-a and RAR-c gene expression increases and the increased ability to metabolize nutrients ensues; 2), through stimulation of the IGF system. It is reported that GnRH pulses amplitude increase together with an increase of gonadal sex steroid output, which in turn lead to higher levels of IGF-1 and vitamin D production. We hypothesize that, by positive feedback, IGF-1 further stimulates the secretion of sex steroids, which in turn potentiated backbone calcification. This could also have influenced the timing of sex differentiation. We assume that the larger GnRH3 neurons size corresponded to a higher GnRH3 expression; this could be related to the higher gene expression levels observed for VDR and IGFs, which in turn could accelerate larval development in terms of growth, backbone calcification and sex differentiation. Zebrafish have two GnRH forms, where GnRH3 has been suggested to elicit gonadotropin-releasing activity and is thought to play a role in sex differentiation. In fact, it was reported that partial ablation of GnRH3 neurons leads to an increased percentage of female fish, while the total ablation leads to an all female population. Sex determination occurs in zebrafish between 21 and 23 dpf. The modulation of GnRH3 observed in probiotic-treated zebrafish could be related to the noteworthy earlier gonad development observed in probiotictreated fish and also to the sex ratio registered at 9 weeks.
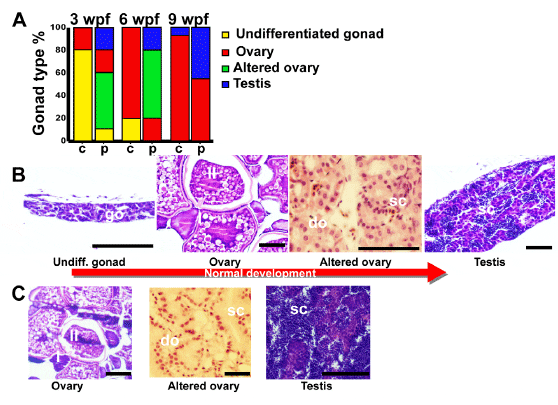
doi:10.1371/journal.pone.0045572.g007
In conclusion, we report that adding L. rhamnosus to the natural zebrafish microflora -from birth to sexual maturity- results into a faster backbone calcification and gonadal differentiation, with altered sex ratios. In the current study we further report that L rhamnosus can also affect the timing of sex differentiation and lead to significantly different sex ratios. Some literature reports highly variable sex ratios in zebrafish populations [64], in fact the unexpected sex ratio found in the control group (over 90% were females) was previously described but we consider noteworthy that the siblings’ population treated with L. rhamnosus developed such a different sex ratio (55% females).
Additional in vitro and in vivo studies should focus on deciphering the signals delivered by L. rhamnosus that have a direct effect on backbone calcification and gonadal development. Further studies are also needed to identify how probiotic administration is involved in sex ratio modulation. These analyses might provide a list of candidate pathways through which the microbiota could affect the physiology of development.
April 2013
Further ReadingYou can view the full list of authors by clicking here. |




