Fish oil (FO) has widely been used as the main lipid source in fish diets, as a source of energy, n-3 polyunsaturated fatty acids (n-3 PUFA) and fatsoluble vitamins (Dosanjh et al., 1998; Bell et al., 2001; Turchini et al., 2005). n-3 PUFA content in aquaculture products is a major factor determining consumer preference because of their beneficial effects on human health (Bayir et al., 2010). Annual worldwide FO production has remained relatively static over the last decade, leading to inflated prices. FO prices reached record levels in early 2011. After a high in February 2011 of more than USD 1.800 per tonne (Peruvian feed grade), prices declined until April 2011 to USD 1.150 per tonne, but have since rebounded to around USD 1.300 FOB (Anonymous, 2011). The current trend is towards the replacement of FO by alternative lipid sources in aquaculture feeds for sustainable aquaculture production.
The effects of complete or partial substitution of fish oil with animal oils on the growth, survival and fatty acid composition in cultured salmonids such as brown trout (Salmo trutta, Turchini et al., 2003; Bayir et al., 2011) and rainbow trout (Oncorhynchus mykiss, Greene and Selivonchick, 1990; Liu et al., 2004) have been studied.
The objective of the present study was to determine the effects of replacement of fish oil by goose fat, sheep tail fat and beef tallow on growth, survival and fatty acid composition of rainbow trout juveniles, Oncorhynchus mykiss.
Materials and Methods
Fish and Experimental Design
Rainbow trout juveniles (Oncorhynchus mykiss) were obtained from the Trout Research and Extension Center of the Fisheries Faculty at the Atatürk University and acclimated to the laboratory conditions for 2 weeks. During acclimation, fish were fed to satiety twice a day with a commercial trout feed. Subsequently, 320 juveniles with an average initial weight of 2.01 g fish-1 were randomly allotted into each of 12 experimental tanks (50x50x70 cm) connected to a closed recirculation water and oxygen auto-supplemented system. The flow rate of water in each tank was 1 L min-1. A diurnal light: dark cycle of 12:12 h was provided by fluorescent lighting. The water temperature was 9-10oC. Triplicate groups of fish were hand-fed to apparent satiety three times daily at 09:00, 12:30 and 16:00 hours for 6 weeks. The fish were weighted at the medium and end of experiment to follow the overall growth.
Experimental Diets
Four experimental casein–gelatin-based diets containing different sources of lipids (Table 1) were formulated to be isonitrogenous (65%) and isolipidic (15%). The different diets contained cod liver oil (CO), goose fat (GF), sheep tail fat (STF) and beef tallow (BT).
Sampling
At the start of the experiment; 3 fish were sacrificed, weighted, immediately frozen in liquid nitrogen and stored -80°C for analyses. After the six-week feeding trial, weight gain (%), specific growth rate (SGR), food conversion ratio (FCR) and survival rate (SR) were calculated. The fish were weighted and one fish from each tank (3 fish treatment-1), were sampled, immediately frozen in liquid nitrogen and stored at -80°C until analyses.
Ingredient and Proximate Composition of the Experimental Diets (% Dry Matter)
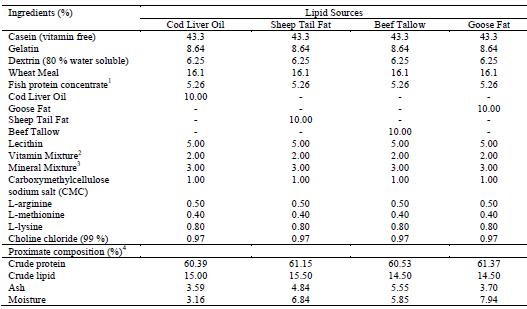
1Concentrate of fish-soluble protein (CPSP 90: crude protein, 82–84% wet weight [WW]; crude lipid, 9–13% WW); Sopropéche S.A., Boulogne-sur-mer, France.
2Roche Performance Premix (Hoffman-La Roche, Inc., Nutley, NJ, USA), composition in per gram of the vitamin mixture: vitamin A, 2645.50 IU; vitamin D3, 220.46 IU; vitamin E, 44.09 IU; vitamin B12, 13 mg; riboflavin, 13.23 mg; niacin, 61.73 mg; d-pantothenic acid, 22.05 mg; menadione, 1.32 mg; folic acid, 1.76 mg; pyridoxine, 4.42 mg; thiamin, 7.95 mg; and d-biotin, 0.31 mg.
3Bernhart Tomarelli salt mixture (ICN Pharmaceuticals, Costa Mesa, CA, USA), composition in g/100 g: calcium carbonate, 2.1; calcium phosphate dibasic, 73.5; citric acid, 0.227; cupric citrate, 0.046; ferric citrate (16–17% Fe), 0.558; magnesium oxide, 2.5; manganese citrate, 0.835; potassium iodide, 0.001; potassium phosphate dibasic, 8.1; potassium oxide, 6.8; sodium chloride, 3.06; sodium phosphate, 2.14; and zinc citrate, 0.133. Five milligrams of Se in the form of sodium selenite was added per kilogram of the salt mixture.
4Crude protein, moisture and ash analyse were conducted using standard procedures (AOAC, 1995).
Lipid and Fatty Acid Analysis
Dietary and whole body lipids were extracted according to the method of Folch et al. (1957). Fatty acid methyl esters (FAME) were obtained on a Hewlett Packard Agilent 6890 N model gas chromatography (GC), equipped with a flame ionization detector and fitted with a DB 23 capillary column (60 m, 0.25 mm i.d. and 0.25 mm) ejector and detector temperature program was 190°C for 35 min than increasing at 30°C per min up to 220°C where it was maintained for 5 min. Carrier gas was hydrogen (2 ml min-1 and split ratio was 30:1). The individual FAs were identified by comparing their retention times to that of a standard mix of FAs (Supelco 37 component FAME mix) and quantification of the individual fatty acids (% of total fatty acids), were made against a C19:0 internal standard from Sigma (USA).
Statistical Analysis
The statistical analyses were performed with SPSS version 10.0 for Windows (SPSS, 1996). Data were presented as mean +/- standard deviation (SD) of the mean. Data were analyzed by one-way analysis of variance (ANOVA). The significant means were compared by Duncan’s multiple range tests at alpha = 0.05 level (n=3).
Results
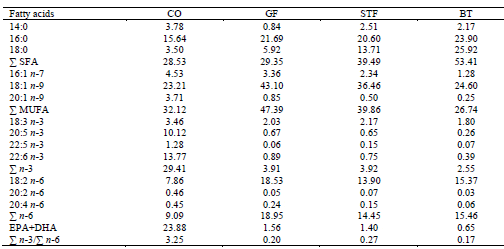
Fatty Acid Composition of the Experimental Diets
Some differences were determined among experimental diets with respect to total and individual fatty acid contents. Levels of total n-3 PUFA, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) content and n-3/n-6 ratio in CO diet were much higher than those in STF, BT and GF diets (Table 2). The level of palmitic acid (16:0) and total saturated fatty acid (SFA) in BT diet, oleic acid (18:1 n-9) and total monounsaturated fatty acid (MUFA) in STF diet and linoleic acid (18:2 n-6) and total n-6 PUFA in GF diet was higher than those in other diets (Table 2).
Growth Performance and Survival
Data on growth performance and SR of rainbow trout juveniles are reported in Table 3. The final weights of fish were not significantly different (7.07-7.28 g) among dietary treatments. SR recorded during the experimental period was 100 % for all treatment groups. Fish increased in weight by around 3.5 fold and SGR and FCR did not show differences in all experimental groups, ranging from 3.01 to 3.07 and from 0.79 to 0.80, respectively.
Fatty Acid Composition
The SFA content was higher in fish fed BT diet (P<0.05) than those fed the CO, GF and STF diets. The 16:0 was the predominant SFA and its highest value was found in the GF and BT diets; 19.49±0.15% and 19.25±0.24%, respectively (Table 4; P<0.05). The 18:1 n-9 was the most abundant fatty acid in all treatment groups. High amount MUFA (49.35±1.14 %) and n-6 PUFA (13.73±0.42 %) content was determined in fish fed the GF diet (Table 4; P<0.05). The fish fed CO diet contained highest levels of total n-3 PUFA, EPA, DHA, linolenic acid (18:3 n-3) while the highest amounts of 18:2 n-6 were found in fish fed the CO and GF diets; 11.20±0.10 % and 11.47±0.53 %, respectively. The n-3/n-6 ratio of CO diet was quite higher compared to that in fish fed the other diets (Table 4, P<0.05).
Fatty Acid Composition of the Experimental Diets (% of Total Fatty Acids)
Discussion
Our results suggest that total substitution of the CO with the GF, STF and BT does not impair growth performance and SR of rainbow trout juveniles (Table 4). Similar results were reported for non salmonids and salmonids in which poultry fat, soybean/corn lecithin, soybean oil, pork lard, beef tallow, goose fat alone or blended with other plant oils and/or animal fats, did not affect the growth parameters and SR in these species (Greene and Selivonchick, 1990; Martino et al., 2002; Turchini et al., 2003; Liu et al., 2004; Noffs et al., 2009; Bayir et al., 2011). In an other publication growth was similar in all oil and yellow grease treated groups but was retarded in comparison to earlier studies (Genc et al., 2005 ) and that canola oil can successfully substitute fish oil in rainbow trout feeds (Dernekbasi et al., 2011). However, an influence was found in the fatty acid profile of whole body (Table 4), which is consistent with previous studies for rainbow and brown trout (Genc et al., 2007, Turchini et al., 2003; Liu et al., 2004; Bayir et al., 2011).
Animal fats such as lard or poultry fats are generally richer in SFA and in the case of poultry fat, have an appreciable amount of the MUFA (mainly 18:1 n-9) (Huang et al., 2008). Goose fat generally contains higher MUFA and PUFA and lower SFA than other poultry species (Wezyk et al., 2003). Similarly, the lowest SFA amount was determined in fish fed the GF diet (Table 4).
The fatty acids of 16:0 and 18:1 n-9 in this study were the most abundant of saturates and monoenes, respectively, contributor with 58-70 % of total SFA and 73-84 % of total MUFA. This might indicate that these fatty acids are the main source of energy and the primary fatty acids selectively incorporated into membrane phospholipids with n-3 HUFA (Lee and Cho, 2009).
Growth Parameters and Survival in Rainbow Trout Fed the Experimental Diets for 6 Weeks

Data are presented as means ± SD (n = 3); 1 IBW, Initial body weight (g/fish); 2 FBW, Final body weight (g/fish); 3WG, Weight gain (% g/100 g) = 100 x (Final body weight – Initial body weight)/Initial body weight; 4SGR, Specific growth rate (%/day) = 100 x [ln (final body weight) – ln (initial body weight)]/feeding days; 5FCR, Food conversion ratio = dry feed intake (g)/weight gain (g); 6SR, Survival rate = 100 x (Final fish number/Initial fish number)
Fatty Acid Content of Whole Body Total Lipids in Rainbow Trout Fed Diets Containing Different Lipid Sources1
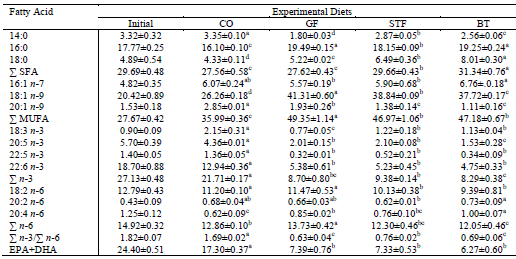
<fontsize="1">1Means with different superscript letters in a row are significantly different (P<0.05).
While the highest total n-3 PUFA, 18:3 n-3 and EPA+DHA levels and n-3/n-6 ratio were found in fish fed the CO diet, the maximum total n-6 PUFA was determined in fish fed GF diet. However, 18:2 n-6 amounts were similar in fish fed the CO and GF diets (Table 4). Many freshwater fish, including salmonids, can convert 18:3 n-3 to 20:5 n-3 and then to 22:6 n-3; by the same enzymatic pathways of desaturation and elongation, they can convert 18:2 n-6 to 20:4 n-6 (Bell et al., 2001). This may explain the higher levels of HUFAs in the whole body of fish than its diets. Moreover, the finding here showed that low level of HUFAs in fish fed animal fats directly related with their low levels in diets.
In conclusion, the results of this study suggest that total substitution of fish oil with animal fats impair nutritional value of the fatty acid composition of rainbow trout juveniles by providing lower EPA+DHA levels, decreasing total n-3 PUFA levels and n-3/n-6 ratio. Moreover, in order to maintain good health, a high weight gain and fertility, fish should receive a diet of adequate values as well as a balanced composition of proteins (amino acids), fats (fatty acids), vitamins and minerals. Animal fats were unbalanced especially in terms of fatty acid profiles due to low level HUFAs. It has been reported that the use of unbalanced feeds in terms of fatty acid composition as the cause of high, pathological level of intestinal accumulation of lipids (Gisbert et al., 2005). But, animal fats in this study did not impair growth performance and survival rate of rainbow trout juveniles as a result of a six-week trial. Therefore, long-term studies with weights closer to the commercial size are also required.
March 2013




