It’s not good enough to accept the well-worn cliches about the benefits of aquaculture. There are still huge improvements to be made and it’s important for researchers to help the industry push the boundaries – for example, by revealing the benefits of seaweed in aquafeeds and the unmet potential of numerous species of herbivorous fish.
What inspired me to take up aquaculture
I was born in Penn ar Bed (Brittany, France) and spent most of my childhood between the family dairy farm inland and the sea, once we moved to the coast. I have been in love with farming on land and with the ocean since I can remember but I only put the two together when I arrived in Australia in 2008 to complete my BSc. I then had my first aquaculture lecture with Dr Nicholas Paul at James Cook University (Townsville, Queensland) and I knew straight away that this was for me.
So, after my first semester at JCU, I changed my major from marine science to aquaculture. During my degree, I volunteered at, and then worked in, a commercial barramundi (Lates calcarifer) hatchery at JCU for three years and realised during that time that, although farming practices had significantly improved since the early days of aquaculture, there was still a lot of room for improvement (fish growth rates, survival and environmental footprint to name a few). This curiosity and desire to continuously improve aquaculture practices and know-how led me to commercially-driven research and, ultimately, to taking up a PhD at the University of the Sunshine Coast (USC) with the Seaweed Research Group.
There is no doubt in my mind that aquaculture can help with food security. It is the fastest growing food producing industry and 50 percent of the fish we consume are now farmed. That is great, but we need to make sure this does not come at a cost. There are two main limitations in the way we grow fish at the moment: 1) reliance on wild-sourced ingredients such as fishmeal and fish oil to farm carnivorous fish and 2) disease outbreaks costing the industry over $6 billion a year (Stentiford et al., 2017). A lot of effort is and has been spent on replacing wild-caught ingredients in the diet of carnivorous fish, with some very promising solutions for the future (Hua et al., 2019). But wouldn’t changing the species of fish we grow be easier than trying to change the diet a fish has been accustomed to for 10-20 million years? Can we farm fish down the food chain in the sea the same way we farm herbivorous livestock on land?

A gametophyte of Asparagopsis taxiformis © Valenitn Thepot
Room for improvement
Although aquaculture is the fastest growing food-producing sector, disease outbreaks are a clear limiting factor to its sustainable development. Putting the financial impact of disease outbreaks aside, about one out of ten fish grown in aquaculture is lost due to disease and will not make it to the consumer’s plate. Additionally, the current methods for treating and preventing disease and parasite outbreaks tend to revolve around veterinary drugs, including antibiotics and other chemotherapeutics. The use of such treatments can have detrimental effects on the environment, the treated fish and – ultimately – it may also have direct or indirect human health implications (Okocha et al., 2018). Although aquaculture uses very small amounts of antibiotics compared to other primary industries, antibiotic resistance was described by the World Health Organisation, during the Covid-19 pandemic, as “one of the biggest threats to global health, food security, and development today” (WHO, 31/07/2020). I have no doubt that antibiotics and other chemotherapeutics serve as an important tool to maintain the welfare of farmed fish, but there is equally no doubt that we need to find alternatives and wean the sector off them.
The fact that antibiotic resistance already claims 700,000 lives each year and has been forecast to kill as many people as cancer by 2050 (10 million annually) acted as my alarm bell. We need to act now and find alternatives to treat and prevent sick fish. This is why the first thing I did during my PhD was to look at what natural alternatives are used to treat/prevent fish diseases in aquaculture.
How seaweed can help
I read multiple scientific publications on probiotics, prebiotics, synbiotics, immunostimulants (eg β-glucans), plants as dietary supplements that could bolster the immune system of fish and ultimately make the fish more resistant to pathogens, reducing and at times eliminating the need to treat fish with traditional chemotherapeutics. In that literature search, seaweed was an ingredient that kept coming back with some very promising results. To confirm my hunch, I decided to compile all the immune (and growth) related data from all 142 published studies which investigated the use of seaweed as a fish immunostimulant. This exercise resulted in a spreadsheet with just under 20,000 entries. I then re-analysed those data and published my work as a meta-analysis review in Reviews in Aquaculture (Thépot et al., 2021a).
In this review, I found that dietary seaweed supplements significantly improved fish immune responses and ultimately made them more resistant to disease when exposed to a pathogen challenge. This review also highlighted that most of the studies investigating the use of seaweed as an immunostimulant for fish only focused on one seaweed species, with the maximum of three seaweed species being tested in a single trial. Considering there are >11,000 species of seaweed globally, with a range of different bioactive properties, I was surprised to have only 34 different seaweed species represented in the 142 reviewed studies. In addition, only two studies out of 142 looked at the effects of seaweed dietary supplements in marine herbivorous fish – species that would naturally encounter and eat seaweed in the wild.
I was already interested in herbivorous fish because of their low-trophic level and low/no requirement for wild-caught fish in their diets, but this finding highlighted the need to explore what seaweed could actually do to the immune system of herbivorous fish. Do they get less of an immune response since they eat seaweed on a daily basis?

Figure 1: mottled rabbitfish (Siganus fuscescens) © Valentin Thepot
Selecting a promising fish species
Based on those insights, it was relatively easy to come up with the aims of the first feeding trial I conducted in my PhD: 1) to test as many seaweed species as possible (out of >11,000 seaweed species) to find the best performing species and 2) screen them for their immunostimulatory potential in a marine herbivorous fish: the mottled rabbitfish Siganus fuscescens.
I chose to work on this particular fish species because of its low-trophic level, but it also has tasty white flesh and, like other rabbitfish species, is capable of producing long chain highly unsaturated fatty acids (omega-3s) from short chain fatty acids. So, unlike its carnivorous counterparts (like salmon and sea bass) it does not require fishmeal and fish oil in its diet. S. fuscescens also has a wide geographical range in the Indo-West Pacific, as it is found from the north of Japan to the south of Australia, and is thus able to cope with temperature from 17°C to 31°C. The warming of the oceans due to climate change is already putting pressure on fish farmers growing temperate species with limited thermal ranges. The robustness of S. fuscescens in regards to some of the consequences of climate change is another reason why I wanted to work with this species.
In that trial, I investigated 11 seaweed species from the three groups (Rhodophyceae, Chlorophyceae and Phaeophyceae), which were collected around the Sunshine Coast or cultured by the USC Seaweed Research Group at the Bribie Island Research Centre, where I built my experimental setup and conducted all my feeding trials (Figure 1). In that trial, I also compared the different seaweed supplements to products, which I encountered in my literature search, and that are used either commercially or at the research level to promote fish immunity: β-glucan (Hilyses), sodium alginate, astaxanthin (Haematococcus pluvialis) and spirulina (Arthrospira platensis).
All supplements were added dry, at 3 percent by weight in commercially available fish pellets (Native Range, Ridley) and were fed to the rabbitfish at 3 percent body weight per day, twice a day. After two weeks of feeding, the fish were harvested, euthanised and blood was collected to assess their innate immune response. The innate immune system is the first line of defence in animals, and in ectotherm vertebrates like fish, the adaptive immune system is often sluggish, taking weeks to reach full potency. For this reason, the innate immune system is a key line of defence in fish when pathogens have been recognised by the fish immune cells.
Immune responses
In the blood of the fish I assessed the lysozyme activity (the enzyme liked with the destruction of gram positive bacteria), phagocytic activity (macrophages and other cells that are capable of engulfing and killing antigens), haemolytic activity (~35 proteins in the blood that form an attack membrane complex on the cell surface of antigens thus neutralising them) and the respiratory burst activity of leukocytes (white blood cells producing cytotoxic compounds to kill antigens).
Out of the four innate immune parameters of interest the most significant response was observed for the haemolytic activity (Figure 2), with the fish fed the red seaweed Asparagopsis taxiformis and the brown seaweed Dictyota intermedia. These showed four-fold increase and two-fold increases in their haemolytic activity respectively, compared to the control fish (Thépot et al., 2021b). This result with A. taxiformis was a clear indication of the potency of this species, but if a natural immunostimulant is to be used commercially then it should not have any detrimental effects on the growth and feed conversion ratio of the fish.
A follow-up trial
This is why in my next trial I used this seaweed and its methanolic extract to test whether 1) the dietary supplement had any growth or food efficiency effects and if 2) the methanolic extract with concentrated seaweed bioactives could outperform the dry seaweed. To do so I bred and reared S. fuscescens
using adapted standard marine larval rearing protocols to have juvenile fish (2g; Figure 3), which would be fast growing and on which I could test the two above points. Because when we talk dietary supplements, the dose must also be considered, this is why in that second trial I decided to explore 1.5 percent (half dose) inclusion, 3 percent (same dose as the first trial) and 6 percent (double dose).
After four weeks on the different diets offered at 3 percent body weight per day, the juvenile S. fuscescens receiving the double dose A. taxiformis extract diet outperformed the other fish with regards to both growth and feed efficiency (Thépot et al., 2021c; Figure 3).
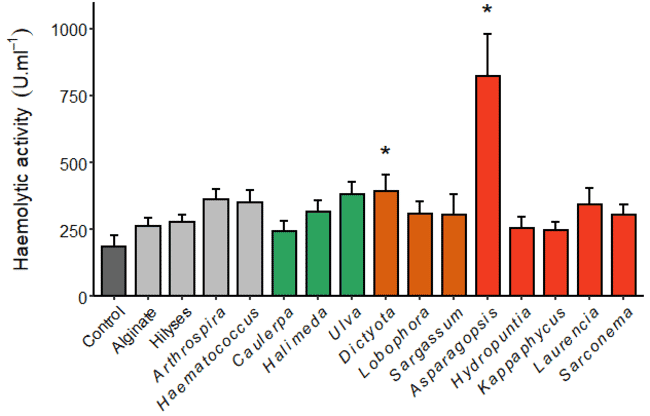
Figure 2: Haemolytic activity (left) of the fish fed diets supplemented with different seaweed and immunostimulants (the asterisks indicate statistical significance).
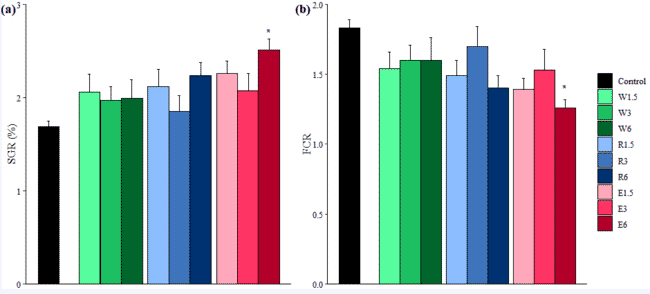
Figure 3: Juvenile S. fuscescens (1 month old; left) and specific growth rates (a) and food conversion ratio (b) of juvenile S. fuscescens fed dry A. taxiformis (‘whole’: W), A. taxiformis methanolic extract (‘Extract’: E) and the residual biomass from the extraction (‘Residue”: R) at 1.5 percent, 3 percent and 6 percent dietary inclusion.
From those two trials I knew that A. taxiformis supplements could promote the growth and improve feed efficiencies and the immune response of the mottled rabbitfish. By that stage I was wondering if those results were rabbitfish-specific. Would I get similar responses in a completely different fish, maybe a temperate freshwater carnivore?
A shift to salmon
More questions = more trials! So, with that in mind the last feeding trial in my PhD investigated the dietary effects of A. taxiformis supplements (dry and extract) in Atlantic salmon (Salmo salar) parr.
I found that, after four weeks of feeding the salmon ad libitum with the three seaweed supplemented diets, the fish were significantly heavier than the control fish (Thépot et al., 2022) and this was especially so for the fish fed the double dose seaweed extract (Figure 4). Albeit lower than for the control fish, the seaweed supplements had no statistically significant effects on the FCR of the fish.
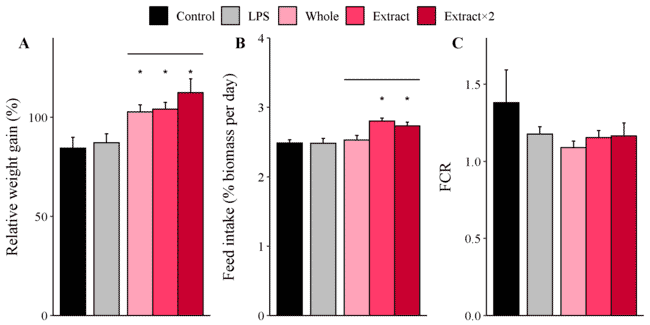
Figure 4: Relative weight gain, feed intake and FCR (top) of Atlantic salmon parr fed the unsupplemented control diet (‘Control’), lipopolysaccharide from E. coli (‘LPS’ at 0.01 percent), A. taxiformis as whole seaweed (‘Whole’ at 3 percent inclusion) as extract (‘Extract’ at 3 percent) and double dose extract (‘Extract×2’). Haemolytic activity of Atlantic salmon fed the different diets for 2 and 4 weeks (middle).

© Valentin Thepot
Although scientific publications are the usual currency in academia this project also produced two international patents, one on the immune boosting properties of A. taxiformis in fish and one on its growth promoting effects. These facilitated the establishment of a collaborative partnership with one of Australia’s most important aquaculture business. The patents were also filed because, regardless of the clear positive effects of A. taxiformis in fish during this project, there must also be a clear economic incentive for an uptake of this technology at scale.
Conclusions
This PhD journey has been extremely rewarding, with multiple publications (scientific and patents) but the most rewarding element was to be able to shine the light on two potential solutions – low trophic level marine fish and seaweed functional ingredients – to two important issues in aquaculture: 1) reliance on wild sourced ingredients for aquafeeds and 2) disease outbreaks.
We only have one planet, with limited and connected resources, so I hope to see more research and commercial interest in the use of seaweeds as functional ingredients for farmed fish and in the development of novel low trophic aquaculture candidate fish.
For my part, I am still working with Siganus spp. and helping colleagues overseas, including in Indonesia, under the Australian Centre for International Agricultural Research project ‘Accelerating the development of finfish mariculture in Cambodia through south-south research cooperation with Indonesia’.
I am in the process of transitioning from academia to join the Anindilyakwa Land Council on the remote Groote Eylandt in the Northern Territory. In my position of aquaculture coordinator, I will help develop a sustainable aquaculture industry to facilitate the transition from mining operations towards a more long-term solution for this remote community. I will keep on working on different marine species, including seaweed, to continue on my path of improving the productivity and environmental footprint of this industry. I’d love to ultimately make this blue revolution a greener one.




