The international sea urchin industry relies on the production of marketable quality gonads, which are sold largely for consumption in the sushi restaurant trade. Gonads that are large in size, contain few to no gametes, have a firm texture, and are bright yellow or orange in colour, are regarded as the most commercially valuable (Robinson, Castell & Kennedy 2002; Shpigel, Marciano, McBride & Lupatsch 2004).
Tripneustes gratilla is a fast-growing shallowwater echinoid, occurring throughout the waters of the tropical Indo-Pacific with isolated populations found in warm temperate Indo-Pacific regions (Dworjanyn, Pirozzi & Liu 2007; Rahman, Tsuchiya & Uehara 2009). This species can produce high quality gonads with excellent market acceptance, and is one of the most commercially important sea urchin species in countries, such as Japan (Rahman et al. 2009), where it is regarded, along with tuna, lobster and abalone, as premium seafood. Demand for high quality sea urchin gonads has, however, led to overfishing of natural populations of this and other echinoid species (Rahman et al. 2009). Consequently, many countries are now examining the feasibility of sea urchin aquaculture to supply the demand from future markets (Lawrence, Olave, Otaiza, Lawrence & Bustos 1997; Pearce, Daggett & Robinson 2002a,b; Hammer, Hammer, Watts, Lawrence & Lawrence 2006; Cook & Kelly 2007) and there is also considerable interest in the cultivation of T. gratilla in South Africa, where it occurs naturally on the north-eastern coast. However, in order for echinoculture to be economically viable, high quality feeds need to be formulated to promote fast growth rates and improve the market acceptability of urchin gonads.
Although Tripneustes species are mostly grazers of sea grasses in tropical regions (Dy, Uy & Corrales 2002; Vaitilingon, Rasolofonirina & Jangoux 2003) and macroalgae (Lawrence & Agatsuma 2001) in more temperate regions, such as in South Africa, the use of wild-harvested macroalgae/ sea grass alone as feed for cultured urchins, as well as their inclusion into prepared diets, are unlikely to be commercially viable for large-scale commercial echinoculture. Disadvantages of using naturally harvested macroalgal diets include limited stocks along the Southern African coast; restricted harvesting in certain areas; temporal and seasonal variation in quality and/or quantity; and the negative environmental effects that arise from harvesting large quantities of seaweeds from natural populations (Anderson et al. 2003; Anderson, Rothman, Share & Drummond 2006; Troell, Robertson-Andersson, Anderson, Bolton, Maneveldt, Halling & Probyn 2006; Anderson, Rand, Rothman & Bolton 2007). The primary disadvantage in using natural diets is, however, the relatively low protein content of most naturally occurring seaweeds, with the consequence that they do not support maximal somatic and gonadal growth of sea urchins (Cook & Kelly 2007). For example, the dominant local kelp Ecklonia maxima which is used extensively in abalone culture as a supplement to formulated feeds has a crude protein content of 11–12% of dry weight throughout the year (Smith 2007). In contrast, formulated feeds are considered to be better than natural foods as they can be formulated to a standard composition and promote maximal production (Lawrence et al. 1997). In addition, they are generally considered less expensive, particularly when taking into account differences in food conversion ratios between seaweeds and formulated feeds (Troell et al. 2006).
Over the last decade numerous studies have demonstrated that artificial diets can enhance somatic growth and gonad yield of sea urchins (Lawrence et al. 1997; Olave, Bustos, Lawrence & Carcamo 2001; Shpigel, McBride, Marciano, Ron & Ben-Amotz 2005), and protein has been identified as the main contributing factor for this enhancement (Pearce et al. 2002a,b; Pearce, Daggett & Robinson 2004). In nature, sea urchins must ingest and process large quantities of macroalgae and/or sea grass to meet their nutritional requirements for protein (Hammer et al. 2006), possibly explaining why administration of macroalgal diets alone typically produces urchins with lower growth rates and smaller sized gonads when compared with urchins fed diets formulated using animal-derived material (Cook, Kelly & McKenzie 1998; Fernandez & Boudouresque 1998, 2000). Hammer et al. (2006) demonstrated that an artificial diet containing a moderate protein level of approximately 20% is used most efficiently by sea urchins, resulting in enhanced consumption rates, survival, specific growth rates and gonad production efficiency, when compared with a diet containing low levels of protein (<9%). These findings are supported by several other studies, which also indicate that a moderate dietary protein level of approximately 20% is most efficiently utilized by sea urchins (Akiyama, Unuma & Yamamota 2001; Pearce et al. 2002a; Schlosser, Lupatsch, Lawrence, Lawrence & Shpigel 2005).
Other components of an artificial feed which affect gonad yield and quality include pigment concentration (especially carotenoids), feed stimulants, feed binder type and feed shape (Plank, Lawrence, Lawrence & Olvera 2002; Pearce et al. 2004; Hammer et al. 2006; Cook & Kelly 2007). Inclusion of small quantities of macroalgae, particularly the brown fucoid seaweed Sargassum linearifolium, in artificial diets fed to T. gratilla has been shown to enhance palatability (Dworjanyn et al. 2007). Carotenoid pigments, mainly β-carotene , which is synthesized by macroalgae, are the source of the red, orange or yellow gonad colouration of sea urchin gonads (Agatsuma, Sato & Taniguchi 2005), and addition of β-carotene to artificial feeds has been shown to improve gonad colour of cultured urchins (Pearce et al. 2002a; Robinson et al. 2002; McBride, Price, Tom, Lawrence & Lawrence 2004; Shpigel et al. 2005). However, despite these potential benefits, the effects of a protein-rich formulated feed supplemented with varying amounts of a particular macroalga on the somatic and gonadal growth of T. gratilla have not yet been investigated. A preliminary study demonstrated that T. gratilla consumed significantly higher amounts of Ulva rigida when offered four seaweed species [Ulva rigida, Ecklonia maxima (kelp), Porphyra capensis and Gigartina polycarpa] in paired consumption tests (Scholtz 2008). As South African Tripneustes generally prefer Ulva and because farm-grown Ulva is available in large quantities from local aquaculture facilities (Bolton, Robertson-Andersson, Shuuluka & Kandjengo 2009), Ulva was selected as an additive for an experimentally formulated urchin feed.
The aim of this study was to determine the effects of incorporating varying levels of the algae Ulva into an artificially formulated feed, with the specific aim of increasing gonad mass and improving gonad colour and quality of the sea urchin Tripneustes gratilla. Ulva was incorporated into the formulated diets to act as both a natural feeding stimulant (Dworjanyn et al. 2007) as well as a source of β-carotene ; an important carotenoid pigment associated with gonad colouration. Ulva lactuca has been shown to contain between 25 and 45 μg g -1 [fresh weight (FW)] of β-carotene (Bischof, Hanelt & Wiencke 2002), whereas the total carotenoid content of farmed Ulva rigida and Ulva lactuca has been reported as high as 10% [dry weight (DW)] (Shuuluka 2011). Urchins fed the various diets were then assessed on a monthly basis to record somatic growth, gonadal growth and a number of gonad quality factors, such as colour, texture, gonad somatic index and the dominant maturity stage of urchins in each experimental group.
Results
Urchin survival and somatic growth
Tripneustes gratilla survival rates over the course of the study were high and did not vary significantly between diets, ranging from 92.5% for animals fed the 0U diet to 97.5% for animals fed fresh Ulva (FU, data not shown).
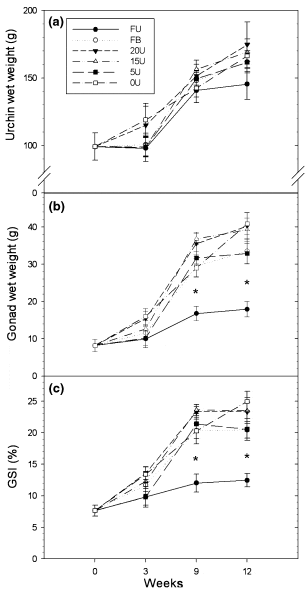
Somatic growth, determined using urchin wet weight, increased significantly (one-way ANOVA, P ≤0.05) within all dietary treatment groups over the 12-week experimental period (Fig. 2a). Overall, the mean wet weight (±SE) of urchins across all treatment groups increased from 99.08 ± 10.15 g at the beginning of the study to 163.13 ± 4.07 g at the end of the study. Urchins fed the artificial diets showed an average gain in wet weight of 68.2% over the course of the 12- week study compared with a 46.7% increase in wet weight for urchins fed fresh Ulva. There was, however, no significant difference (one-way ANOVA, F5,42 = 0.813, P = 0.546 for the 12-week sampling date) in urchin wet weight between any of the treatment groups by the end of the study period. Similar results were recorded for urchin test diameter (data not shown).
Gonad growth and quality
Gonad growth and yield
Urchins fed the formulated feeds had a significantly greater gonad wet weight and GSI by week 9 and at the end of the experiment when compared with urchins fed exclusively on a diet of fresh Ulva (one-way ANOVA; P≤0.001 for gonad wet weight and GSI for the 12-week sampling date) (Fig. 2b and c). By week 9, urchins fed formulated feeds had already achieved a 190 ± 12.09% (mean ± SE) increase in GSI, from the initial starting value, compared with a 57.3% increase in GSI for animals fed exclusively with fresh Ulva over the same time period (Fig. 2c). There was, however, no significant difference between the GSI of urchins fed the different formulated feeds at any stage of the experiment.
Figure 2 Mean (a) urchin wet weight, (b) gonad wet weight and (c) gonad somatic index (GSI) of Tripneustes gratilla fed with four artificial diets, fresh Ulva or a mixed diet consisting of fresh Ulva and a basal feed over a 12-week grow-out period in recirculating seawater systems. Data represents mean ± SE of eight replicates per treatment group. *(P < 0.05, Tukey test) represents a significant difference in the means of urchins fed the artificial or mixed diets from the means of urchins fed fresh Ulva. Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva.
Gonad colour
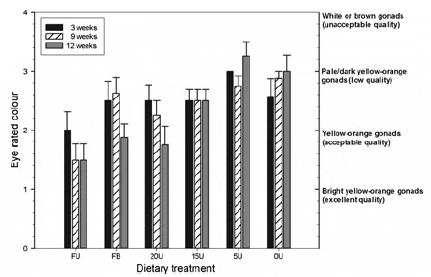
Mean eye rated gonad colour (Fig 3) ranged from 1.8 to 3.4 for urchins fed the formulated feeds, whereas urchins fed with fresh Ulva produced lower colour ratings which fluctuated between 1.4 and 2. At the end of the feeding trial, urchins fed the 20U diet and fresh Ulva had comparable colour ratings that were not significantly different from one another (Kruskal–Wallis, P > 0.05). In contrast, the gonad colour of urchins fed the remaining three artificial diets (0U, 5U, 15U) was significantly higher, or worse in terms of market acceptance, than the fresh Ulva treatment (Kruskal –Wallis, H5 = 22.37, P = 0.0004 for the 12-week sampling date) by the end of this study.
Figure 3 Mean eye rated (ER) gonad colour of Tripneustes gratilla fed with four artificial diets, fresh Ulva or a mixed diet consisting of fresh Ulva and a basal feed over a 12-week grow-out period. Data represents the mean ± SE of eight replicates per treatment group. Gonad colour was visually ranked in categories ranging from most desirable to unacceptable. The categories were allocated numbers, which were ranked as follows: (1) bright yellow-orange gonads (excellent quality); (2) yellow-orange gonads (acceptable quality); (3) pale yellow-orange or dark yelloworange gonads (low quality) and (4) white or brown gonads (unacceptable). Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva.
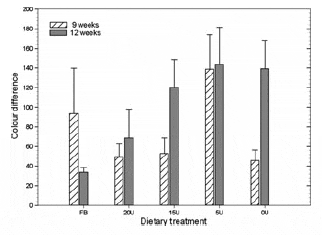
Gonad lightness (L*) values ranged from 57.88 to 62.44 and were unaffected by diet, with no significant difference recorded between any of the dietary treatments by the end of this study (Fig. 4a). Likewise, gonad redness (a*) values did not vary significantly between treatment groups over the course of the study and ranged from 11.17 to 15.22 (Fig. 4b). In contrast, mean gonad yellowness (b*) values decreased with the decreasing Ulva content of each diet and by week 9 urchins in the 5U treatment group showed significantly lower gonad b* values when compared with the FU treatment group (Fig. 4c; one-way ANOVA; F5,42 = 3.891, P = 0.001). By the end of the feeding trial, the 15U, 5U and 0U treatment groups had significantly lower b* values (F5,42 = 5.92, P = 0.0003) compared with the FU treatment group. In contrast, urchins in the 20U and FB treatment groups produce gonads with similar yellowness values that were not significantly different from urchins fed a diet of fresh Ulva by the end of the study. The total difference in gonad colour, calculated using the formula from McBride et al. (2004), between urchins fed fresh Ulva and the various artificial diets support the latter findings, with total gonad colour values increasing as the Ulva content of each diet decreased (Fig. 5). The 20U and FB treatment groups did, however, produce urchins with gonads that were similar in colour to urchins fed fresh Ulva, and these two treatments had significantly better colouration (one-way ANOVA, F = 2.901, d.f. = 39, P = 0.035 for the 12-week sampling date), in terms of their market acceptance, compared with the 15U, 5U and 0U treatment groups by week 12.
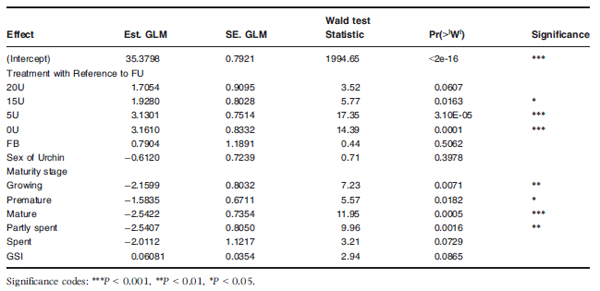
In addition, a Fitted Generalized Estimating Equations General Linear Model (geeglm) revealed that gonad colour (L*, a*, b*) was significantly influenced by dietary treatment and gonad maturity, as gonads in the FU treatment group were significantly better coloured, in terms of their market acceptance, than those in the 15U (P = 0.016), 5U (P = 3.1e-05) and 0U (P = 0.0001) treatment groups (Table 4). The 20U and FB treatment groups, however, did not vary significantly in terms of gonad colour when compared with the FU diet. Gonad maturity also contributed to and significantly affected gonad colour, with gonads in a recovering stage having similar coloured gonads to those in a spent stage. Whereas gonads in the growing (P = 0.0071), pre-mature (P = 0.0182), mature (P = 0.0005) or partly spent (P = 0.0016) states had significantly better coloured gonads than those in a recovery or spent state (Table 4).
Figure 4 Mean gonad (a) L* (lightness), (b) a* (redness) and (c) b* (yellowness) values of Tripneustes gratilla fed with four artificial diets, fresh Ulva or a mixed diet consisting of fresh Ulva and a basal feed over a 12- week grow-out period. Data represent the mean ± SE of eight replicates per treatment group. *(P < 0.05, Tukey test) represents a significance differences in the means of urchins fed the artificial or mixed diets from the means of urchins fed fresh Ulva. Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva.
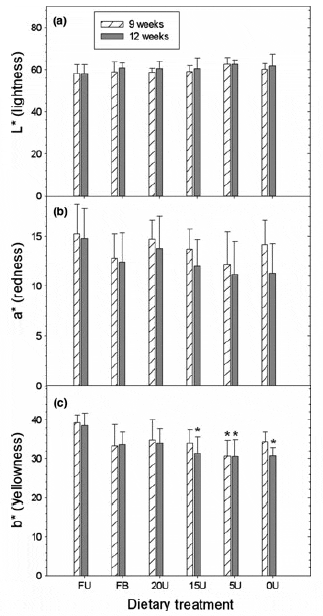
Figure 5 The total difference in spectrophotometer rated gonad colour of Tripneustes gratilla fed with the four artificial diets or a mixed diet (consisting of fresh Ulva and a basal diet) from the gonad colour of urchins fed with fresh Ulva. Data represent the mean ± SE of eight replicates per treatment group. Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva.
Gonad texture and firmness
Mean gonad texture ratings at the end of the study period ranged from 1.75 to 2.88 and all treatment groups consisted of gonads with two distinct gonad segment halves with at least some visible follicle separation. There were, however, no significant differences in gonad texture ratings between any of the treatment groups at each of the sampling dates. Similarly, mean gonad firmness ratings did not differ significantly between treatments at the end of the study period and ranged from 2 to 3, indicating that gonads were soft– firm in their appearance.
Histology
Histological analysis of sea urchin gonads revealed a significant degree of variability in overall gametogenic development, and therefore gonad maturity, between sampling dates within all treatment groups (Kruskal–Wallis, H = 72.68, d.f. = 3, P = 6.565e-007), however, there were no significant differences between the different dietary treatment groups at each sampling date (Fig. 6). At the beginning of the feeding trial, the gonads of approximately 50% of urchins were in a recovery stage, whereas the remaining 50% were split evenly with 25% being partly spent and 25% being completely spent (Fig. 6a). Thereafter, there was a fairly consistent progression in gonad maturity, with gonads developing or maturing throughout the study period and accumulating nutritive phagocytes, which were stored and then used to produce gametes.
Table 4 A Fit Generalized Estimating Equations General Linear model (geeglm) analysis showing the significant interactions between dietary treatment, sex of an urchin, certain maturity stages and Gonad Somatic Index (GSI) when predicting spectrophotometer rated gonad colour values (L*, a*, b*) of Tripneustes gratilla fed with four artificial diets, fresh Ulva and a mixed diet over a 12-week grow-out period. All non-significant interactions were excluded from the table. Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva
Although gonad maturity did not differ significantly between the treatment groups, a General Linear Model analysis revealed (Table 5) that the density of NP is significantly affected by dietary treatment, gonad maturity and sex of the urchin. During week 9, diet type significantly (F5,37 =2.743, P = 0.033) affected the storage of nutrients and therefore the density of NP’s (Fig. 7) within the gonad. Urchins in the 20U treatment group had significantly more densely packed NPs compared with the 5U (P = 0.005) and 0U (P = 0.025) treatment groups, tending to indicate that the addition of Ulva to prepared diets may affect cell density. Gonad maturity was also shown to have an effect on NP density with gonads in the recovery stage having significantly denser packed NP’s compared with gonads in a partly spent (P = 0.006) or spent stage (P = 0.007) (Table 5). Sex was also shown to significantly (P < 0.00001) affect NP density within the gonad as female gonads had more densely packed NP’s compared with male gonads. Nutritive phagocytes density therefore appeared to show a general trend of decreasing as gonads developed gametes and the gonads mature.
Discussion
The artificial diets formulated and tested in this study have been shown to significantly increase gonad yield in the sea urchin Tripneustes gratilla when compared with urchins fed a diet of fresh Ulva. The GSI of urchins fed artificial diets in this study ranged from 20.05% to 24.96% (≈200% increase) at the end of the 12-week study, whereas urchins fed exclusively with a natural algae diet had significantly lower GSI values, reaching a maximum of just 12.5% (64% increase) within the same time period. These findings are consistent with the results of previous studies that have demonstrated the effectiveness of artificial feeds for enhancing the growth and development of sea urchins, with artificial diets having successfully increased the GSI of cultured Evechinus chloroticus (James 2006; Phillips, Hamid, Silcock, Sewell, Barker, Weaver, Delahunty & Bremer 2009; Phillips, Bremer, Silcock, Hamid, Delahunty, Barker & Kissick 2010), Psammechinus miliaris (Pantazis, Kelly, Connolly & Black 2000), Paracentrotus lividus (Spirlet, Grosjean & Jangoux 2000; McBride et al. 2004; Schlosser et al. 2005; Shpigel et al. 2005), Strongylocentrotus droebachiensis (Walker & Lesser 1998; Pearce et al. 2002a, 2004), Lytechinus variegatus (Hammer, Hammer, Watts, Desmond, Lawrence & Lawrence 2004; Taylor, Powell, Watts & Lawrence 2009) and Loxechinus albus (Lawrence et al. 1997; Olave et al. 2001) when compared with wild caught individuals or individuals fed natural diets. This study also demonstrated that cultured T. gratilla fed ad libitum with the macroalga Ulva achieved significantly higher GSI values (up to 12.5%) when compared with the GSI values (maximum of 4.9%) reported for T. gratilla collected from the wild (Muthiga 2005). However, even though the artificial diets tested in these studies produced large gonads, many of the gonads were pale and unmarketable. In contrast, we demonstrated that artificial diets supplemented with 20% (w/w) dried Ulva (20U) can produce bright yellow, marketable gonads which do not differ significantly in colour in the experimental conditions from those produced on fresh Ulva.
Figure 6 The mean number of Tripneustes gratilla (n = 8) of both sexes allocated to each maturity stage over the 12-week grow-out period. Gonads of urchins fed the four artificial diets, fresh Ulva and a mixed diet (consisting of fresh Ulva and a basal feed) were processed for routine paraffin histology at (a) week 0, (b) week 3, (c) week 9 and (d) week 12 to assess the amount of gametogenic activity in the gonads and categorized as (1) recovery; (2) growing; (3) premature; (4) mature; (5) partly spent or (6) spent. Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva.
Table 5 A generalized Linear Model (GLM) showing the significant interactions between wet gonad weight, dietary treatment, sampling date, gonad maturity and sex of urchin when predicting the density of nutritive phagocytes within the gonads of Tripneustes gratilla fed with four artificial diets, fresh Ulva and a mixed diet over a 12-week grow-out period. All non-significant interactions were excluded from the table. Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva
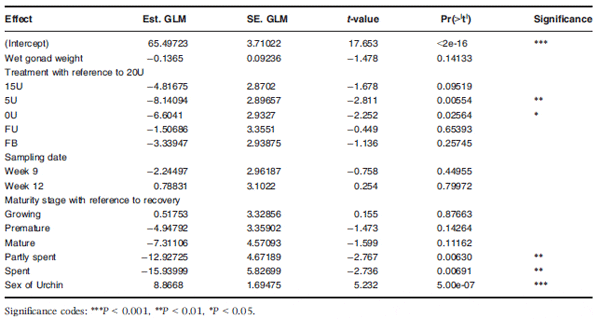
Figure 7 Mean nutritive phagocyte density (NP) (percentage ± SE, n = 8) within the gonads of Tripneustes gratilla fed with four artificial diets, fresh Ulva or a mixed diet consisting of fresh Ulva and a basal feed over a 12-week grow-out period. Treatment groups: FU = fresh Ulva; FB = fresh Ulva and the basal diet; 20U = 20% Ulva; 15U = 15% Ulva; 5U = 5% Ulva; 0U = 0% Ulva.
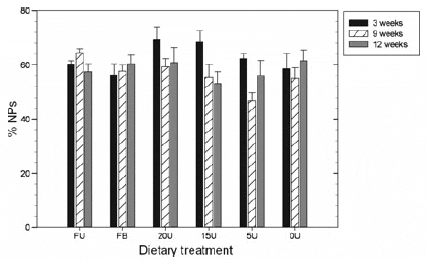
Table 6 Gonad growth, expressed as a per cent increase in gonad somatic index (GSI) per week, of a variety of sea urchin species fed with artificial and natural macroalga feeds
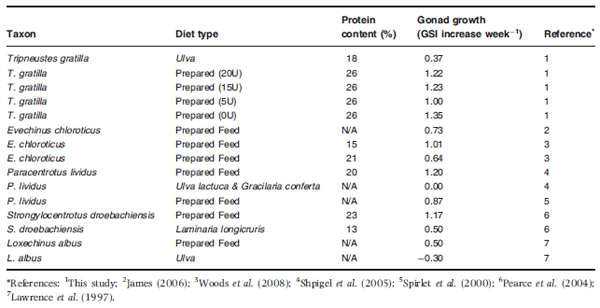
The gonad growth rates obtained from urchins fed formulated feeds in this study compare quite favourably to those in other studies in the literature for formulated and natural feeds (Table 6). On average, T. gratilla fed formulated feeds had a 1.2% increase in gonad somatic index per week over the course of this study, which is comparable to the growth rates reported by Pearce et al. (2004) and Shpigel et al. (2005) for Strongylocentrotus droebachiensis and Paracentrotus lividus respectively. Moreover, weekly gonad growth rates on feeds formulated in this study were 19 and 87.5% higher than the growth rates of Evechinus chloroticus in a similar study using two different formulated feeds currently used in commercial echinoculture (Woods, James, Moss, Wright & Siikavuopio 2008). These reports would tend to suggest that some of the feeds currently formulated for commercial echinoculture do not achieve maximum GSI gain and therefore can be improved.
The high GSI values recorded in the literature for urchins fed artificial diets compared with those fed natural algal diets have been attributed, primarily, to differences in protein levels between the feeds. As protein is one of the most expensive components of an artificial feed, it is essential to determine the optimal levels of protein required to maximize growth. Previous studies have determined that a moderate dietary protein level of approximately 20% is most efficient for maximizing the gonad growth of sea urchins (Akiyama et al. 2001; Pearce et al. 2002a; Schlosser et al. 2005). In this study, all artificial diets were formulated to contain roughly equal crude protein concentrations (≈26%), so that the only variable differing between the prepared diets was the inclusion of different amounts of Ulva or Ulva additive. Ulva used in this study had a crude protein content of 18.31%, which is significantly lower than the protein content of the prepared diets (≈26%); possibly explaining the enhanced growth and development of urchins fed prepared diets compared with fresh Ulva. It could, however, also be argued that the enhanced growth of urchins fed the prepared diets in this study may be attributed to differences in consumption rates. Consumption rates for T. gratilla fed the 20U diet were significantly higher compared with animals fed the 5U and 0U diets (data not shown). However, no significant differences in urchin wet weight or gonad wet weight were recorded between these treatment groups (Fig. 2). Also, each urchin consumed, on average, approximately 3.5 g of Ulva (wet weight) per day compared with 1.5–2.0 g of each prepared feed (dry weight) per day (data not shown). As Ulva has a moisture content of approximately 80% (Shuuluka 2011), this equates to approximately 0.7 g of Ulva (dry weight) per urchin per day. These findings suggest that the high protein content and increased consumption of the prepared feeds may have collectively contributed to the enhanced growth of urchins fed the prepared diets in the present study, although other factors, such as feed shape and texture should also be considered. Moreover, our consumption data also suggests that the inclusion of high quantities of dried Ulva into a prepared feed may act as a feeding stimulant.
Schlosser et al. (2005) suggest that digestible energy may also be a limiting factor in naturally available diets, such as Ulva, as they showed that a prepared diet with a crude protein content of 23% produced significantly higher GSI values compared with a natural diet of Ulva lactuca with a crude protein content of 35%. In their study, the prepared feed and fresh Ulva had gross energy values of 19.39 and 13.39 MJ kg -1 respectively. Schlosser et al. (2005) believe that the difference in energy content between these two feeds resulted in enhanced growth and gonad development with the prepared feed. The higher energy content of the prepared feed used in that study is similar to the results recorded in the present study, where the prepared feeds in the current study had gross energy contents between 15.49 and 17.18 MJ kg -1, whereas the natural Ulva diet had a gross energy content of 9.44 MJ kg -1. Both protein and energy contents of Ulva in this study were therefore shown to be suboptimal, as the artificial feeds contained 65% more energy and 44% more protein than Ulva. The low gross energy, crude protein content and consumption of Ulva utilized in this study makes it difficult to determine which of the three factors, if not all, may have contributed to the low GSI values recorded for urchins fed fresh Ulva compared with those fed artificial diets.
Gonads of commercial quality need to be acceptable in terms of colour, which in the past has proven to be more difficult to achieve using artificial diets compared with natural diets (Senaratna, Evans, Southham & Tsvetnenko 2005; Shpigel et al. 2005). Differences in colour between animals fed natural and artificial diets have been attributed mainly to the lack of carotenoid pigments generally contained within artificial diets (Robinson et al. 2002; Shpigel et al. 2005; Shpigel, Schlosser, Ben-Amotz, Lawrence & Lawrence 2006; Symonds, Caris-Veyrat, Kelly & Young 2007). In this study, the addition of Ulva to our formulated diets acted as a natural source of carotenoid pigments, with Ulva rigida and Ulva lactuca having an average total carotenoid content of 6.7% and 7.3% DW respectively (Shuuluka 2011). Echinenone, which is synthesized from β-carotene, is responsible for the yellowish-orange colour of high quality roe (Pearce et al. 2002a; McBride et al. 2004; Shpigel et al. 2005). We demonstrated that gonad colour is dependent on the inclusion ratio (0%, 5%, 15% and 20% w/w) of Ulva within the diets, with higher inclusion rates promoting better coloured gonads. The FU diet, as expected, proved to be the most successful at producing gonads of marketable colour, and this is most likely due to the high concentration of β-carotene contained within this diet compared with the artificial ones. Indeed, the FU diet produced gonads which differed significantly in eye rated colour from all other diets used in this study with the exception of the 20U diet. As eye rated colour may be quite subjective, a non-subjective colour measure was also employed which gave three colour values, namely lightness (L*), redness (a*) and yellowness (b*). In our study there were no significant differences in gonad lightness (L*) or redness (a*) between any of the dietary treatments. However, the yellowness (b*) values recorded in this study varied significantly between the sampling dates and dietary treatments. By the 9th week, FU gonad b* values varied significantly from only the 5U diet. However, by the 12th week the FB and 20U diets did not vary significantly from the FU b* values, whereas diets which contained less Ulva (15U, 5U and 0U diets) produced gonads which were less yellow. These findings reinforce the importance of carotenoid pigments in sea urchin diets for increasing gonad quality and suggest that both the 20U and FB diets can successfully produce gonads that are not significantly different in terms of colour from gonads of urchins fed a natural diet of Ulva. Robinson et al. (2002) reported similar results for gonad yellowness (b*) values during the course of their study, as yellowness decreased significantly over time in gonads of animals fed artificial diets, whereas wild urchins (Strongylocentrotus droebachiensis) feeding on natural algae diets had an increase in gonad yellowness over time. Aside from the obvious commercial interests in higher profits obtained for better coloured gonads, it has been suggested that there are several other advantages to adding carotenoid pigments to prepared sea urchin diets, as pigments within actively growing tissue can act as important antioxidants, are involved in protein stabilization, aid in egg production and provide ultraviolet protection for sensitive tissues (George & Lawrence 2002; Robinson et al. 2002).
In this study, texture and firmness ratings for all treatment groups and sampling dates did not vary significantly over the study period and generally fell within an acceptable range for sale on commercial markets. However, a gonad factor which differed significantly between treatment groups and sampling dates was the density of NPs contained within gonads. There was a trend of decreasing gonad NP volume with decreasing Ulva content, and we demonstrated that the 5U and 0U diets had significantly less densely packed NPs, ranging from 46.8% to 62.2% over the course of the study, compared with the FU, FB, 20U and 15U diets, which ranged from 53.1% to 68.7% and did not differ significantly in NP density from each other. Gonads in a recovery phase were also show to have significantly more densely packed NPs compared with gonads in a partly spent or spent stage, whereas urchins in a growing, premature or mature stage did not differ significantly. This result is, however, not surprising, as development of gonads from recovery to mature stages is characterized by nutrient accumulation and gamete development, whereas immediately before and after spawning nutrients have been used up and most gametes have been released, resulting in a decrease in gonad NP density. Our results support findings by Bo¨ttger, Devin and Walker (2006), who found that S. droebachiensis in early pre-gametogenesis have an increased volume of NP’s within the gonad compared with gonads in later maturity stages. Histological analysis of gonads over the course of this study did, however, reveal no significant difference in gonad maturity between the different dietary treatments. Gonad maturation followed a similar trend in all treatment groups with gonads accumulating nutrients within NPs, followed by the development and storage of gametes.
In conclusion, we have clearly demonstrated that prepared diets (26% crude protein content) can significantly increase gonad yield of urchins within 9 weeks, compared with urchins fed only a natural diet of fresh Ulva. More importantly, we have demonstrated that when these artificial diets are supplemented with 20% (w/w) dried Ulva, marketable quality gonads can be produced which do not differ significantly in colouration from the gonads of urchins fed with fresh seaweed. Therefore, there is clearly potential for artificial diets containing Ulva to support the commercial cultivation of Tripneustes gratilla in South Africa, and for the local industry to become part of an already successful and very lucrative international echinoculture industry.
May 2014


