The results showed that ex vivo exposure of C. divergens at 108 CFU ml-1 did not cause cell damage to the intestine tract of Atlantic salmon. Furthermore, prior provision of dietary prebiotic elevated the ability of C. divergens to adhere to the epithelium or mucus layer in the proximal intestine, where culturable heterotrophic bacterial levels (which were identified as C. divergens) were elevated by 234% compared to the control. This effect was not apparent in the distal intestine.
The ability of isolated carnobacteria from the ex vivo experiment to inhibit growth of two fish pathogenic bacteria (Yersinia rückeri and Aeromonas salmonicida ssp. salmonicida) was assessed.
Extracellular products from all 11 of the isolated carnobacteria strains, plus the type strain Carnobacterium inhibens CCUG 31728, inhibited the in vitro growth of Y. rückeri. However, only extracellular products from C. divergens isolate 57 inhibited the growth of A. salmonicida ssp. salmonicida.
Introduction
Today it is generally accepted that the three major routes of infection in fish are through: a) skin, b) gills and c) the gastrointestinal (GI) tract. The GI microbiota, including lactic acid bacteria (LAB), have been suggested to be important in fish health and it has been suggested that the autochthonous gut bacterial community may be responsible for controlling the colonization of potential pathogens by adhesion competition and production of antagonistic compounds. If the GI tract is involved as an infection route, scientists have address whether probiotic bacteria are able to adhere to and colonise mucosal surfaces and outcompete endogenous bacteria and pathogens.
Investigating these topics effectively in in vivo models can be difficult as they are time consuming and have high cost. Furthermore, as the EU has recommend reductions of in vivo experiments and the numbers of animals used in experiments (Revision of the EU directive for the protection of animals used for scientific purposes [Directive 86/609/EEC]; 8th of September 2010), attempts have been made to use alternative ex vivo methods (e.g. the Ussing chamber, everted sack and intestinal sack methods).
The first aim of the present study was to investigate possible effects of a prebiotic feed on epithelial histology and indigenous GI tract microbiota in the proximal intestine (PI) and distal intestine (DI) of Atlantic salmon. Furthermore, the same effects, including morphological changes of epithelial cells after ex vivo exposure of the intestinal tract to Carnobacterium divergens, a probiotic bacterium, are investigated by light microscopy and electron microscopy. The result of Carnobacterium exposure is of high importance to evaluate as translocation and cell damage are negative criteria when evaluating the use of probiotics in endothermic animals as well as in fish.
The 2nd aim of the present study was to evaluate the bacterial community of the PI and DI of salmon fed control or prebiotic diets, before and after ex vivo exposure to probiotic bacteria, in order to investigate if the indigenous GI tract microbiota is modulated by the different treatments. Finally, we addressed the issue as to whether carnobacteria isolated in the ex vivo studies were able to inhibit in vitro growth of the pathogenic bacteria Yersinia rückeri and Aeromonas salmonicida ssp. salmonicida.
Material and Methods
Fish husbandry
Two hundred and forty vaccinated Atlantic salmon (Salmo salar L.) were held at the EWOS Innovation AS Research Station,Dirdal,Norway. The average weight at the start of the experiment was350 g. Two hundred and forty fish were distributed equally (i.e. 40 fish per tank) into six tanks supplied with500 litersof sea water and two diets were offered (i.e. triplicate tanks per diet). The control diet and prebiotic diet had the same ingredient composition (Table 1) and differed only in the inclusion of 0.2% EWOS prebiosal® in the prebiotic diet. EWOS prebiosal®, is described as a multi-component prebiotic specifically designed for salmonid fish; more detailed information about the composition of EWOS prebiosal® is not available for commercial reasons. Feeding was conducted twice a day with duration of 2.5 hour between each feeding for a period of 15 weeks. During the feeding period the water temperature and salinity ranged, with season, from 5.3-12.9°C and 26.7-30.9 gl-1.
Table 1: Dietary Formulation and Chemical Composition of the Experimental Diets
The samplings were carried out at two different points: at the start (week 0) and at the end of the trial (week 15). An overview of the different treatments and groups is listed in Table 2.
Table 2: Experimental Treatments Applied to Atlantic Salmon Intestine Fed Control and Prebiotic Diets
Probiotic Bacteria
The probiotic bacterium used in this experiment was Carnobacteriumdivergens strain Lab01 originally isolated from juvenile Atlantic salmon fed a commercial diet. The bacteria were stored in glycerol-containing cryotubes at -80°C and inoculated into tryptic soy broth (Difco, USA) with glucose (10g l-1) and NaCl (10g l-1), viz. TSBgs medium. After approximately 24 hours of pre-inoculation at room temperature with an agitation of 190rpm, 1% of the pre-culture was transferred to new TSBgs medium and growth (same growth conditions as above) was measured by optical density for evaluation of the growth cycle (data not shown). Bacterial viability was confirmed by plating bacterial suspensions on tryptic soy agar (Difco) + glucose (15g l-1) and NaCl (15g l-1) (TSAgs) plates. The results obtained from this study were used to calculate the bacterial concentration in the experimental bacterial solutions.
Ex Vivo Exposure to Bacteria
Three fish were randomly selected from two of the tanks fed each diet and killed with a blow to the head. The entire intestine, from the last pyloric caeca to the anus, was removed aseptically and intestinal content was gently squeezed out, before the intestine was flushed three times with sterile saline solution (0.9 % NaCl), in order to remove the allochthonous gut bacteria. The posterior end was tightly tied with cotton thread before filling (ca. 1.5ml) with the appropriate assay solution (Table 2), tying the anterior end and suspending the sealed intestinal tube in sterile saline solution. The intestinal sacks were then incubated at 10ºC for 1 hr.
After incubation the intestine was cut open, the contents discarded and flushed three times with sterile saline solution.
Post Ex Vivo Bacterial Assays
Samples for bacteriology from each segment from the first sampling point (groups 1 and 2) were prepared by homogenizing1 gramof intestinal tissue (PI or DI) in 1 ml sterile saline using a Stomacher (Seaward Laboratory,UK). Gut samples for bacteriology from the second sampling (groups 3-6) were prepared by gently scraping off mucus with a sterile scalpel. Thereafter, the segments were weighed. Both the homogenates and mucus were used to create serial ten-fold dilutions which were spread plated (100µl) on TSAgs plates and incubated at 6ºC for up to 1 week to determine viable counts of culturable heterotrophic bacteria.
After sub-culturing on TSAgs to achieve pure cultures, phenotypic bacterial identification (Gram stain, colony morphology, oxidase - and catalase tests and glucose fermentation) was carried out on random colonies from all plates containing between 10-300 colonies. A total of 168 bacterial strains were isolated from the two sampling points.
16S rRNA Characterization of Isolates
The bacterial DNA was isolated following the protocol from a commercial kit (DNeasy Blood and Tissue,Qiagen,USA). Specific treatment for Gram-positive and Gram-negative isolates was carried out according to the manufacturer’s instructions. Template-DNA was diluted to a concentration of approximately 20-30 ng µl-1 using Milli-Q water. The PCR mix constituted of 8µl of template-DNA, 36µl Milli-Q water, 5µl 10x buffer F511, 0.25µl dNTP, 0.25µl27Fforward primer, 0.25µl 1492R reverse primer and 0.25µl DNA-polymerase yielding a total volume of 50µl. PCR thermal cycling consisted of initial denaturation of 94ºC, followed by 35 cycles of 94ºC for 20 s, 53ºC for 20 s and 72ºC for 90 s with a final extension step of 72ºC for 7 min. To verify PCR products, samples were run on gel electrophoresis. The PCR-products were desalted by mixing 20µl of PCR-product with 40µl of 100% ethanol and 2µl of 3M NaOAc (pH 5.3) and vortexed well. Samples were then incubated on ice for 30 min followed by centrifugation for 20 minutes at14,000 gusing an Eppendorf Microcentrifuge Model 5417R. The supernatant was removed and pellet washed in 100µl of 80% ethanol and centrifuged for another 5 min at14,000 g. The supernatant was removed and the pellet dried at room temperature for 60 min. The pellet was then resuspended in 30µl of Milli-Q water. Purified PCR products were sequenced as described elsewhere.
The resultant nucleotide sequences were submitted to a BLAST search in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to retrieve the closest known alignment identities for the partial 16S rRNA sequences. Gene sequences that showed higher than 95% similarity to a genus or species in GenBank were categorized accordingly.
In Vitro Growth Inhibition Of Pathogens By LAB Isolated Form The Ex Vivo Studies
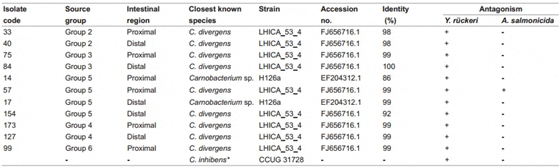
Eleven randomly chosen LAB isolated from the intestinal tract after ex vivo exposure and one type strain, Carnobacterium inhibens (CCUG 31728), were tested for antagonistic effects against two different fish pathogens. The pathogenic bacteria used in the present investigation were Yersinia rückeri (CCUG 14190) and Aeromonas salmonicida ssp. salmonicida (Ass 4017). C. inhibens (CCUG 31728) was used as a positive control as previous investigations have demonstrated that this strain has an inhibiting effect towards V. anguillarum and A. salmonicida. In vitro growth inhibition of the two fish pathogens by the twelve LAB was tested using a microtitre plate assay described in detail by Ringø and co-authors. This method has been used in two recent studies. The pathogenic bacterial levels at the start of assays were 106 cells ml-1. Positive in vitro growth inhibition was defined when no growth (turbidity < 0.05 at optical density; OD600 nm) of the pathogen was detected. Sterile growth media and the pathogens were used as controls. Growth (at OD600) of the pathogens without addition of sterile supernatant of LAB was approximately 0.6. Measurements were carried out each hour using an automatic plate reader (Bioscreen C,Labsystems,Finland).
Histology
Samples for light microscopy (LM) and transmission electron microscopy (TEM) were collected by excising approximately 5mm from the posterior part of the PI and DI. The samples were immediately fixed in McDowells fixative and stored at 4ºC until processing. TEM and LM samples were processed as described elsewhere Morphological observations were made from multiple micrographs (8) from each intestinal region from two fish within each group. The following morphological parameters were observed; detached microvilli, enterocytes detached from the basal membrane, disintegrated cell junctions, presence of goblet cells, presence of absorptive vacuoles and presence of intraepithelial lymphocytes.
Result
Bacterial Levels After Ex Vivo Exposure
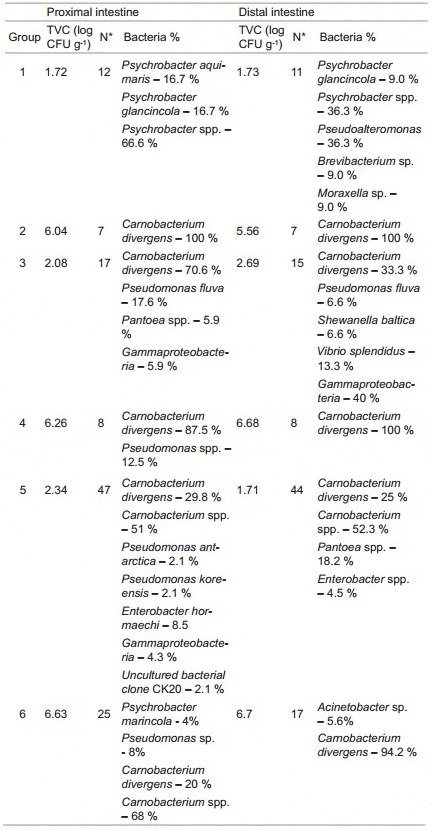
The adherent bacterial levels, as determined by using a stomacher (groups 1 and 2) or by the collection of mucus (and subsequent weighing of the segments the mucus was removed from) (groups 3 – 6), did not seem to differ which indicates that the different sampling methods were similarly effective. Table 3 presents an overview of the autochthonous bacterial levels isolated from each segment and each group exposed to either saline or C. divergens. All values are expressed as log colony forming units (CFU) g-1. Autochthonous bacteria isolated from intestines of fish fed the control diet at the experimental start and exposed to saline was approximately log 1.7 CFU g-1 in both PI and DI, while the number of bacteria isolated from intestines of fish exposed to C. divergens was log 6.04 CFU g-1 in PI and log 5.56 CFU g-1 in DI. After 15 weeks of feeding slightly higher values were present in PI of fish fed the prebiotic diet post exposure to saline or C. divergens compared to fish fed the control diet. Indeed, the bacterial level in the prebiotic fed fish intestine exposed to C. divergens (group 6) was 234% greater than that of the control fed fish intestine exposed to C. divergens (group 4). In both dietary groups, a similar bacterial level (~log 6.70 CFU g-1) was detected in DI exposed to C. divergens. However, a higher bacterial level (log 2.69 CFU g-1) was observed in the DI of control fed fish exposed to saline than that of prebiotic fed fish (log 1.71 CFU g-1).
Table 3: Cultruable Heterotropic Bacterial Levels (log CFU g-1 wet weight) and Identity (as determined from phenotypic characteristics and 16S rRNA sequence analysus) Obtained from Different Groups after the Ex Vivo Assay
Isolation and Identification of Bacteria after Ex Vivo Exposure
A total of 168 bacterial strains were isolated from the two samplings. Among these, 40 isolates were isolated from the first sampling point and 128 isolates were isolated from the second sampling point. All isolates were tested for morphology and biochemical properties (colony morphology, Gram-testing, oxidase - and catalase tests and glucose fermentation).
One hundred and eleven isolates were further identified by partial sequencing of the 16S rRNA gene. Isolates not identified by 16S rRNA gene sequencing but showing similar biochemical and physiological properties to those isolates identified by 16S rRNA genes were defined as “– like”. Table 3 provides an overview of the different bacterial species isolated in each experimental group.
Week 0 (experimental start)
Microbiota of fish fed control diet and intestines exposed to saline (group 1): Analysis of the adherent microbiota in the PI of fish fed the control diet and exposed to sterile saline (group 1) revealed that all isolates belonged to the genus Psychrobacter. Of the 12 strains isolated from the PI of this group, 2 strains showed 96 % similarity to Psychrobacter aquimaris, 2 strains were identified as Psychrobacter glacincola while 8 strains were identified as Psychrobacter spp. - like.
The DI of fish exposed to saline at the first sampling point showed a more diverse community which consisted of 4 different bacterial genera. Of these, 10 strains were indentified to genus level and 1 strain was identified to species level. The bacteria identified to genus level belonged to Pseudoalteromonas, Psychrobacter, Moraxella and Brevibacterium, while the last strains showed high similarity (98 %) to Psychrobacter glacincola.
Microbiota of fish fed control diet and intestines exposed to C. divergens (group 2): All bacteria isolated from PI and DI of fish exposed to C. divergens at the first sampling (group 2) were identified as C. divergens. This observation indicates that C. divergens are able to adhere to the intestinal mucosa in both segments.
Week 15
Microbiota of fish fed control diet and intestines exposed to saline (group 3): After 15 weeks of feeding on the control diet, the isolated strains (17) from the PI exposed to saline were dominated by C. divergens; 70.6% of the isolates were identified as C. divergens, 17.6% were identified as Pseudomonas fulva, 5.9% belonged to Pantoea spp. while 5.9% of the isolates were identified as members of the class Gammaproteobacteria. The bacteria isolated from the DI were identified as C. divergens, 2 strains as Vibrio splendidus, 1 strain as Shewanella baltica, 1 strain as Pseudomonas fulva and 6 other strains were identified as Gammaproteobacteria.
Microbiota of fish fed control diet and intestines exposed to C. divergens (group 4): In the intestine of fish fed the control diet for 15 weeks and exposed to C. divergens, the identified bacterial strains isolated from both PI and DI were dominated by C. divergens. Only 1 strain, identified as Pseudomonas spp., isolated from the PI of 1 fish did not belong to the species C. divergens.
Microbiota of fish fed prebiotic diet and intestines exposed to saline (group 5): The intestine exposed to saline of fish fed the prebiotic diet for 15 weeks showed higher diversity compared to the other groups exposed to saline (groups 1 and 3). Of the 47 strains isolated from the PI, 14 were identified as C. divergens, 1 as Pseudomonasantarctica, 1 as Pseudomonas koreensis, 4 as Enterobacter hormaechei and 1 as uncultured bacterial clone CK20. The remaining isolated strains were identified as members of the Carnobacterium and Acinetobacter genera. The dominant bacteria in the PI of this group belonged to carnobacteria (81%) and 30% of total isolates were identified as C. divergens.
The bacterial composition of the isolates from the DI of fish fed the prebiotic diet for 15 weeks were relatively low in diversity. Of the total number of strains isolated (44) from the DI exposed to saline 34 were identified as Carnobacterium, 8 strains showed high similarity (%) to Pantoea spp. and 2 strains belonged to the genus Enterobacter.
Microbiota of fish fed prebiotic diet and intestines exposed to C. divergens (group 6): In group 6, fish fed the prebiotic diets for 15 weeks and exposed to C. divergens, the isolated strains in the PI were dominated by C. divergens and C. divergens - like strains. Of the 22 carnobacteria isolated, 5 were identified as C. divergens by 16S rRNA sequencing while 17 isolates were identified as C. divergens – like. Three other isolates were identified as members of the genera Pseudomonas (2 strains) and Psychrobacter (1 strain). Of the 17 strains isolated and identified from the DI of group6, C. divergens and C. divergens – like strains dominated with only one isolate, which showed high similarity (99 %) to Acinetobacter spp., not belonging to this species.
Microscopical Analyses
Light microscopy (LM): All LM micrographs, both from PI and DI of the prebiotic groups (5 and 6) showed no morphological differences compared to the control feeding regime (groups 1 - 4). All intestinal sections examined appeared normal and healthy; no signs of detached enterocytes, necrotic enterocytes, widened lamina propria or necrosis were observed and the number of goblet cells were similar in both treatments (examples are displayed in Figure 1).
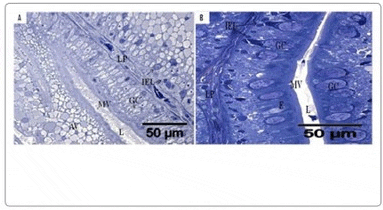
Transmission electron microscopy (TEM): Similar to the observations using LM, TEM revealed no differences between treatments or exposure groups; all micrographs revealed healthy epithelial brushborder, no deteriation of tight junctions was observed and microvilli appeared uniform. The presences of rodlet - like cells (as shown in Figure 2) were present in the PI and DI of all groups. The numbers of rodlet cells present in the PI displayed great differences between individual fish but were always observed in the upper half of the epithelium, above the underlying intraepithelial lymphocytes.

In Vitro Growth Inhibition of Two Fish Pathogens by Extracellular Extracts of LAB Isolated From Ex Vivo Studies
Identification by partial sequencing of the 16S rRNA genes of the eleven LAB strains isolated from the ex vivo experiments and subsequently used in the in vitro pathogen antagonism assays are displayed in Table 4. The results show that growth inhibition of Y. rückeri was obtained from extracellular extracts from all strains of carnobacteria isolated from the ex vivo experiment. However, in vitro growth inhibition of A. salmonicida ssp. salmonicida was only obtained from the extracellular extract of C divergens isolate 57. The extracellular products from the positive control, C. inhibens CCUG 31728, did not inhibit the growth of A. salmonicida ssp. salmonicida.
Table 4: Identification of LAB Strains and Pathogens Antagonistics Activity of Extracellular Products used in the in vitro Pathogens Assays.
Discussion
The ex vivo intestinal sack method has been used in several studies to evaluate possible histological changes in the fish intestine after exposure to high levels of LAB. The result of LAB exposure to the intestine is of high importance as translocation and cell damage have been proposed as important criteria when evaluating the use of probiotics in endothermic animals as well as in fish. Recently, the effect of ex vivo LAB exposure on the gut microbiota in fish was documented, but to the authors’ knowledge the effect of prebiotic supplementation and ex vivo LAB exposure of the fish intestine has not been investigated.
The culturable bacterial levels recovered on TSAgs plates from groups exposed to saline were relatively low, ranging from log 1.72 to 2.34 CFU g-1. These values are low compared to autochthonous levels previously reported in Atlantic salmon and rainbow trout Oncorhynchus mykiss. This is likely due to the thorough rinsing process, 3 times prior and 3 times post probiotic/saline exposure. Culturable adherent bacteria in the PI observed in the prebiotic group exposed to saline (group 5) was log 2.34 CFU g-1. The fact that the value from this group is higher than in control groups exposed to saline water (log 1.72 and 2.08 CFU g-1), might be due to a feeding effect of the prebiotic diet. However, this hypothesis merits further investigations.
Investigations of the qualitative and quantitative bacterial composition of the intestinal microbiota were based on the study of 168 pure cultured bacterial isolates. These isolates were biochemically tested in order to obtain a general classification. From this classification 111 isolates were selected by the lottery method and identified by 16S rRNA gene sequencing analysis.
The bacterial levels observed in groups exposed to saline varied between segments and feeding regime. By comparing different feeding groups it is clear that the indigenous microbiota of the PI were affected by the diet, while the effect of prebiotic feeding on the microbiota was less clear in the DI. In the intestine exposed to C. divergens the average number of bacteria were higher in the PI when the fish were fed the prebiotic diet compared to fish fed the control diet. Whether these findings can be related to a higher C. divergens colonization success in prebiotic fed fish merits further investigations.
The bacterial composition from the PI in control groups, i.e. fish fed control diet, and thereafter exposed to saline were dominated by members of a few genera (Psychrobacter, Carnobacterium and Pseudomonas). Psychrobacter, Carnobacterium and Pseudomonas spp. are commonly reported in the GI tract of fish, and these bacteria have previously been isolated and identified from the GI tract of salmonids. Carnobacterium spp. have often been reported to be components of the gut microbiota of salmonids; indeed, C. maltaromaticum, C. mobile, C. divergens and Carnobacterium spp. have been identified from Atlantic salmon. The consistency of isolation of these species indicates that these might be common core components of the GI microbiome of Atlantic salmon and are likely to be of importance to the host.
The bacterial composition isolated from the DI in the first control group exposed to saline (group 1) were dominated by Pseudoalteromonas spp. and Psychrobacter spp., while in the other control group (group 3) Acinetobacter spp. and C. divergens were the dominant bacteria isolated. All of these listed bacteria have previously been isolated from the intestine of Atlantic salmon. The bacterial composition of the PI observed in the prebiotic fed fish exposed to saline (group 5) showed greater bacterial diversity than that observed from control fed fish exposed to saline (groups 1 and 3).
The majority of the bacteria from the PI of group 5 were C. divergens and Carnobacterium spp., which together accounted for 81% of all the isolated bacteria. The two other bacterial species that were isolated from this group were Pantoea spp. and an unidentified member of the class Gammaproteobacteria. The abundance of culturable adherent Carnobacterium spp. (77% of isolates identified) was higher in the DI of the prebiotic fed fish (group 5), compared to control groups (group 1 = 0% and group 3 = 36%).
These results suggest that the prebiotic supplementation elevates autochthonous Carnobacterium spp. levels, particularly in the DI. To the authors’ knowledge there is very little information regarding the effect of prebiotics on carnobacteria within the GI tract of fish. However, some studies suggest that the carnobacteria populations within the GI tract of salmonids are effected by various dietary factors such as krill meal and oxytetracycline in Atlantic salmon and dietary carbohydrates in Arctic charr (Salvelinus alpinus L.). However, it was observed that the presence of dietary inulin (a prebiotic-type carbohydrate) tended to lower culturable autochthonous carnobacteria levels (by ca. 90%) in the hindgut of Arctic charr and also elevated the proportion of C. maltaromaticum at the expense of C. divergens. These findings suggest that different prebiotics may influence different carnobacteria strains in different fish species.
In all groups exposed to C. divergens in the ex vivo studies the same C. divergens strain was identified to dominate both the PI and DI after exposure. C. divergens levels were in the range of 104-106 CFU g-1 intestine which indicates that the bacteria are able to populate and potentially colonize the intestinal mucus and out-compete other adherent bacteria after only one hour of exposure. These results are in accordance with corresponding studies in that LAB are able to colonize the intestine of Atlantic salmon after one hour exposure.
Despite the plethora of information available on the prebiotic efficacy of elevating probiotic colonization (i.e. synbiotics) in various terrestrial species, little information is available in fish. Further studies should focus on this topic as the present study demonstrated that the presence of the dietary prebiotic, prebiosal®, elevated the proportion of carnobacteria from 71% to 81% in the DI (as well as elevating total bacterial levels, effectively quadrupling the number of carnobacteria) and from 33% to 77% in the PI (although the total bacterial population was lower).
The histological effect of exposing the GI tract of Atlantic salmon to high levels of the C. divergens was investigated by light and electron microscopy. Furthermore, the intestinal effects of feeding a prebiotic diet to Atlantic salmon were evaluated.
Results from LM-investigations in the present study showed no apparent histopathological changes of the epithelium in the PI or DI, after exposure of C. divergens. In particular the micrographs demonstrated that enterocytes showed no signs of junctional rupture from the basement membrane which is in contrast to observations of the PI of Atlantic salmon after exposure to Vibrio anguillarum and A. salmonicida.
TEM observations confirmed the findings observed in LM regarding a lack of histological changes. TEM revealed no observable differences between the groups in respect to the presence of cell debris in the lumen, the amount of mucus, the number bacteria – like particles in the lumen and between the microvilli, disorganized microvilli and disintegrated tight junctions. The enterocytes within all groups displayed normal cell contacts with unaffected tight junctions and zona adherens. The fact that C. divergens did not inflict damage to the intercellular unction is great importantance since the loosening of these junctions contributes to a paracellular port of entry for potential pathogens.
Rodlet cells were present in large numbers in the PI of all groups, while in the DI the number observed were lower. Since groups exposed to C. divergens did not display any clear differences in number of rodlet cells compared to groups exposed to saline, their presence in those groups may therefore not be related to an immunological function towards the exposed bacteria. On the other hand, the role of rodlet cells as immune cells and their large number in the PI compared to the DI may be a defense function towards potential invading bacteria of the PI. Since the PI has been confirmed as being an infection route for pathogenic bacteria by several studies, the role of rodlet cells as immune cells in the PI is possible and warrants further investigation.
The antimicrobial effects of LAB have long been utilized in food preservation by fermentation and several comprehensive reviews have been published on the ability of LAB to produce proteinaceous antimicrobial substances. In fish studies, the antagonistic effect of LAB has been carried out on Gram-negative fish pathogens such as V anguillarum and A salmonicida. In the present study strong growth inhibition of Y. rückeri was recorded from extracellular extracts from late exponential growth phase from all of the eleven Carnobacteria strains isolated from the ex vivo experiments. However, the ability of the isolated strains to inhibit growth of A. salmonicida ssp. salmonicida was only observed from one strain isolated from the PI. The fact that only one (isolate 57) of the 11 strains displayed inhibitory effects towards A. salmonicida ssp. salmonicidais in accordance with the results of Ringø who observed a lack of antagonism when challenging A. salmonicida ssp. salmonicida to extracellular extracts from C. divergens strain Lab01. These results indicate that the production of extracellular products only, might not be sufficient for strains of C. divergens in late exponential growth phase, to inhibit growth of A. salmonicida ssp. salmonicida.
The positive control bacteria, C. inhibens which Jöborn et al. reported to display antagonistic effect against A. salmonicida, showed no sign of antagonism in the present study. This observation therefore indicates that antagonisitic activity of C. inhibens is only effective when cells are actively incubated together or that antagonistic extracellular products are only produced by C. inhibens in the presence of A. salmonicida.
The ability of C. divergens as useful probiotics with effects against Y. rückeri and A. salmonicida have previously been reported in vivo and in vitro. Kim and Austin observed that dietary provision of C. divergens strain B33, isolated from the intestine of healthy rainbow trout, increased survival of rainbow trout against A. salmonicida and Y. rückeri challenge by 60% compared to the control group. Even though strains of C. divergens show antagonistic effects against pathogens, the precise mechanism of action of antimicrobial compounds isolated from fish remains unclear, but suggestions about their ability of penetrating cell walls by forming pores and channels, thus rendering it more fragile and incapable of carrying out normal metabolism has been proposed.
In order to confirm the in vitro probiotic effect of C. divergens against Y. rückeri in Atlantic salmon, further investigations should therefore include in vivo challenges studies. By further applying electron microscopy, the physical interference mechanisms between C. divergens and Y. rückeri in the GI tract might be observed.
Acknowledgements
The authors thank the technical staff at EWOS Innovation AS for feed manufacture, analysis and running the feeding trial and thank Dr. Sigmund Sperstad and Dr. Chun Li, Norwegian College of Fishery Science, University of Tromsø for their inestimable help during 16S rRNA gene sequencing and in vitro growth inhibition. We also thank Randi Olsen and Helga Marie By at the EM department at University of Tromsø, and Anne Nyhaug at Molecular Imaging Centre, Institute for Biomedicine, University of Bergen for their inestimable help during light and electron microscopy analysis. This study was partially supported by grants from the Norwegian MABIT-program (project number AF0038).
August 2013




