Most people will have heard of antimicrobial resistance (AMR), but can you explain what it is?
When we talk about antimicrobial resistance (AMR), it normally refers to bacteria developing resistance to antibiotics, but all types of microbes – including viruses and fungi – can potentially develop resistance to most substances designed to control them.

© Fish Vet Group
In the case of antibiotics, these are medicines which act upon bacterial cells to either kill them or cause them to stop reproducing. They do this by affecting very specific cell processes, unlike disinfectants which destroy bacteria and other microrgansims relatively indiscriminately. This specificity means that relatively minor random mutations in the cell structures and processes upon which an antibiotic acts can quickly result in an individual bacterium becoming less sensitive or even resistant to that antibiotic. Following an antibiotic treatment, these less sensitive bacteria may survive in small numbers and reproduce, and so the subsequent generation of bacteria is less sensitive. With repeated exposure, some bacteria can become entirely resistant to a given class of antibiotics.
It would be easy to imagine that the consequences of antibiotic resistance developing in a particular bacterial pathogen of human beings, animals or fish might be limited to clinical problems treating those diseases with which they are linked, but this isn’t the case at all. Bacteria don’t only develop resistance to antibiotics through direct exposure, but can also acquire resistance from other species of bacteria through various mechanisms, most notably the exchange of small pieces of DNA, called plasmids, which can jump from one species of bacteria to another. Resistance to a particular class of antibiotics in bacterial pathogens of one species could move to those of another, and there is some evidence that this is happening between humans and animals in both directions.
This matters because human and animal diseases are beginning to emerge against which very few - if any - antibiotics will work. If the trend continues, this will have profound implications for the way we live. For humans and animals, including aquaculture species, this will include increasing treatment failure for simple bacterial infections.
How much of an issue is AMR in aquaculture globally?
Firstly, neither AMR in aquatic nor terrestrial farming is a distinct issue from AMR in human health. In fact, AMR is the quintessential “One Health” issue, a concept that has emerged in the last 15 years which recognises that medical, veterinary and environmental health are inextricably linked. In world aquaculture, just as in terrestrial farming, pet health and human medicine, it’s an enormously important issue.
The range of aquaculture species and environments in which we farm them is much more diverse than on land, and the situation across the breadth of these not easily summarised. We see the most AMR where antibiotic use is high, and in too many aquaculture sectors globally antibiotics are overused – most severely in developing countries where their availability is often unregulated. I’ve seen this myself working in fish farms in such developing countries, where without access to diagnostics or advice on correct administration, farmers attempt to treat undiagnosed diseases - some of which may not be bacterial in nature - with antibiotics purchased without the involvement of a vet or other qualified adviser.
By contrast, veterinary colleagues working with terrestrial species are often surprised to learn that Atlantic salmon in Scotland and Norway receive the lowest levels of antibiotic in terms of milligrams per kilogram farmed weight of all major meat producing species in those countries. The vast majority of salmon farmed in these countries will not receive antibiotics at any stage in their cycle. This has been achieved through the development of successful vaccines for the major bacterial pathogens and, critically, an improved focus on husbandry and hygiene. This is a fragile success which should not be taken for granted.

Is there anything unique about AMR in aquaculture?
It is incumbent on all of us involved in farming fish or other species - whether freshwater or marine - to recognise that aquatic environments generally represent a major opportunity for AMR to arise.
On one hand, in aquaculture antibiotics have not been used as “growth promoters” to nearly the same extent as in intensive livestock production. This practice of providing animals non-therapeutic doses of antimicrobials in their diet to achieve improvement in growth rate is now banned in the EU and US, although it still occurs globally and is thought to be a major driver of AMR.
However, certain aspects of antibiotic use in aquaculture can lead to AMR. Aquaculture systems will contain water with large and diverse populations of bacteria, often concentrated in effluent flows. The liquid medium facilitates quite effective exposure of such environmental bacteria to antibiotics, and the same will also mean effective mixing of emergent resistant bacterial populations with other bacterial species and thus the exchange of plasmids conferring resistance. Furthermore, the vast majority of antibiotic treatments for fish and shellfish species are performed in-feed. Most antibiotics are poorly absorbed in the gut by fish, with the majority excreted unchanged. As a result production units such as ponds and tanks represent locations where the exposure of large bacterial populations to antibiotics can be singularly high at times creating potentially ideal conditions for AMR to develop.
Beyond their effect on the target bacteria in the fish, do antibiotics have other effects on fish and their environment?
This is a really good question, and one I think we’re only beginning to understand. Much of the work published in the last few years describing the effects of antibiotics on fish and the biomes of which they are a part brings to mind the famous quote from the Scottish naturalist John Muir: when we try to pick out anything by itself, we find it hitched to everything else in the universe.
Like all animals, including humans, healthy fish exist with a wide abundance of bacteria normally present on their skin, gills and in their guts. The number of different functional genes in these microbiota significantly outnumber those of the fish itself. We now understand that, far from being passengers, these bacteria produce metabolites which play a role in health: they improve digestion, produce micronutrients and augment the immune response. In humans, the gut microbiome even appears to have an effect on the brain, affecting things like mood and cognition. The role these microbiota play in overall fish health will be similarly profound and complicated. Recent work in Tasmania has shown that fish microbiota are closely related to the biofilms of the tanks in which they are kept. Fish health depends on a healthy homeostasis – or balance – of the microbial communities present on and in the fish and its environment. In simple terms, bacterial disease occurs where something disrupts this balance, such as stress, overstocking or poor hygiene.

© David Moriarty
When we treat fish or shellfish with antibiotics, usually in-feed, we don’t simply affect the disease-causing bacteria. We will collaterally expose the entire gut microbiome to the antibiotic, and as the active ingredient is passed then in turn the skin and environmental microbiota. This may select for resistance in gut, skin, gill or environmental bacteria species, or interfere with the role they play in resilience against future disease.
I think it’s likely that, within the span of my career, routine diagnostic work will involve looking at the health and diversity of the fish microbiome through sequencing approaches. As a result we will more frequently think of fish health as a holistic product of this balance of microbes, and be increasingly cautious about the effect of antimicrobials on it.
Are the targets set by the Responsible Use of Medicines in Agriculture Alliance (RUMA) realistic?
RUMA has set targets for UK salmon and trout production of below 5mg/kg and 20mg/kg farmed biomass per year respectively, but the goal for any fish farmer should be to raise fish without antibiotics at all – in that sense our target should always be zero.
I read recently that as early as the 1950s, shortly after the first use of antibiotics in aquaculture, the pioneering Polish fish pathologist Stanislas F Snieszko warned never to rely on antibiotics in the long term. This remains good advice today. Aquaculture units which are inherently reliant on antibiotics – which actively plan for their use rather than strive to eliminate them – are fundamentally unsustainable.
At an industry level, the reality is that in any farming situation – particularly one where the intention is to increase the farmed biomass such as salmon – we will see both emergent (new) bacterial pathogens and familiar pathogens for which we have effective vaccines developing new subtypes for which the same existing vaccines are less effective. Used judiciously, antibiotics are a valuable tool to safeguard fish welfare in those situations – so at least some use can be expected at an industry level.
With respect to the salmon target of below 5 mg/kg, I think this is both ambitious and achievable. Indeed, in 2018 figures, the sector used only marginally more than this: 6.5mg/kg. Because the baseline use is low (by comparison, the UK pig sector target for 2020 is 99 mg/kg) it is important to understand that single instances of sites treating with antibiotics – particularly late in the cycle where the biomass is considerably higher than in freshwater – have the potential to appear to significantly inflate the annual usage. So caution is needed with interpreting numbers this low – the figure will appear to fluctuate despite relatively small changes in usage patterns.
Similarly, I think the UK trout sector’s target of maintenance of use below 20mg/kg is correct and the 2018 figures of 13 mg/kg would compare very favourably with most rainbow trout industries, to judge from my experience from working overseas, although relatively little data is available for comparison. The financial incentive for veterinary pharmaceutical companies to develop trout vaccines is comparatively small in the UK alone, but as the trout sector grows across the Americas, Europe and its bordering countries, the growing market for vaccines will incentivise their research and development. The work to develop a polyvalent vaccine for Flavobacterium psychrophilum at Stirling’s Institute of Aquaculture is exciting and such projects have the potential to further drive down antibiotic use in UK trout production.
How can we prevent AMR in aquaculture?
The short answer is that all of us involved in aquaculture should be striving in practice to eliminate the use of antibiotics. Of course, salmonid aquaculture in Europe is quite different to tilapia farming in North Africa and the scale of that challenge will differ around the world.
Availability of veterinary antimicrobials for fish is an issue. I have read NGO reports for developing countries citing unavailability of veterinary medicines as a major barrier to sustainable growth, only to visit and find antibiotics very freely available outside of any veterinary involvement. Governments need to regulate the supply of antibiotics, but also encourage fast access to diagnostic labs, training and advice on preventative health for their aquaculture sectors. The goal of eliminating antibiotic use in aquaculture entirely may be unrealistic, but in very many countries I believe substantial reductions could be made quickly which would dwarf the incremental improvements we are looking for in the best performing sectors.
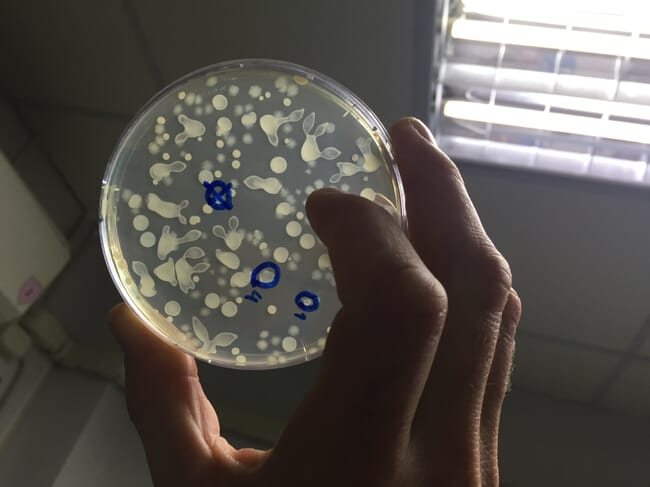
Where antibiotics are used, the use must be based on a correct diagnosis. Where possible, bacterial cultures should be grown and antibiotic sensitivity tested in order to look for the development of resistance and inform the choice of antibiotic. Farmers and their prescribing vets should review all instances where antibiotics have become necessary and ask what preventative health steps can be taken to avoid a recurrence. This is central to the veterinary health planning process, which should look systematically at the biosecurity, hygiene and environment on any aquaculture unit.
Vaccines can be central to preventing bacterial disease, though even the best vaccines can be overwhelmed if there is not attention to all other aspects of disease prevention. Where vaccines are available, farmers have a role to play in pharmacovigilance by reporting instances of disease in vaccinated fish to their vet and the vaccine manufacturers in turn, who are then better able to keep the antigen profile updated against emergent variants of a given bacterial disease.
One interesting emergent approach to tackling bacterial disease in aquaculture is the use of bacteriophages. Bacteriophages are viruses which are naturally occurring pathogens of bacteria. Some of these can be isolated, grown and used to treat bacterial diseases. This work was initially pioneered in the former Soviet Union but was side-lined by the increasing availability of antibiotics following the Second World War. Due to AMR, there is renewed interest in bacteriophages and their application in human and animal health. The recent launch of a bacteriophage product to treat Yersiniosis in salmon in Norway (CUSTUS YRS, ACD Pharma) is major milestone to be celebrated and I look forward to seeing more phage products emerge for aquaculture in the future.




