Omega-3 (also called n-3) long-chain polyunsaturated fatty acids (≥C20; LC-PUFAs) are of considerable interest, based on clear evidence of dietary health benefits and the concurrent decline of global sources (fish oils). Generating alternative transgenic plant sources of omega-3 LC-PUFAs, i.e. eicosapentaenoic acid (20:5 n-3, EPA) and docosahexaenoic acid (22:6 n-3, DHA) has previously proved problematic.
Here we describe a set of heterologous genes capable of efficiently directing synthesis of these fatty acids in the seed oil of the crop Camelina sativa, while simultaneously avoiding accumulation of undesirable intermediate fatty acids. We describe two iterations: RRes_EPA in which seeds contain EPA levels of up to 31 per cent (mean 24 per cent), and RRes_DHA, in which seeds accumulate up to 12 per cent EPA and 14 per cent DHA (mean 11 per cent EPA and 8 per cent DHA). These omega-3 LC-PUFA levels are equivalent to those in fish oils, and represent a sustainable, terrestrial source of these fatty acids. We also describe the distribution of these non-native fatty acids within C. sativa seed lipids, and consider these data in the context of our current understanding of acyl exchange during seed oil synthesis.
Introduction
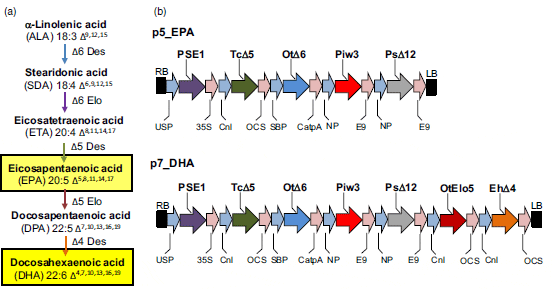
It is now well established that omega-3 LC-PUFAs have critical roles in human health (Saravanan et al., 2010). Currently, the primary dietary source of these fatty acids is marine fish; however, the increasing demand for fish oil (particularly due to the expansion of the aquaculture industry, the major consumer of these oils) places enormous pressure on diminishing marine stocks (Cressey, 2009). Such over-fishing and concerns related to pollution in the marine environment have triggered an urgent search for a completely new source of omega-3 LC-PUFAs (Tocher, 2009). When evaluating any potential new source of these fatty acids, it should be both sustainable and capable of meeting any increased demand. An appealing approach to renewable supply of omega-3 LC-PUFAs is metabolic engineering of a crop plant with the capacity to synthesize these fatty acids in seeds. The scalability of agriculturebased production systems, in conjunction with modest running costs, highlights the potential of transgenic plants as ‘green factories’ for the synthesis of desired compounds (Dyer et al., 2008). An oilseed crop such as Camelina sativa is an attractive host for such metabolic engineering, based on its low input costs and ease of transformation (Nguyen et al., 2013). Introduction of the omega-3 LC-PUFA biosynthetic pathway into a crop demands the addition of multiple genes (for primary synthesis and to direct the flux of substrate and biosynthetic intermediates) that require co-ordinated tissue-specific expression in the developing seeds of the transgenic host (Cheng et al., 2010; Haslam et al., 2013). It is for this reason that reconstruction of this pathway represents the cutting edge of metabolic engineering in transgenic plants (Wu et al., 2005; Ruiz-Lopez et al., 2012). However, effective accumulation of the target fatty acids, eicosapentaenoic acid (20:5 n-3, EPA) and especially docosahexaenoic acid (22:6 n-3, DHA), at levels comparable to those in fish has proved technically challenging to date, despite many attempts to optimize their synthesis (see Figure 1a for a simplified representation of biosynthetic pathway; Ruiz-Lopez et al., 2012; Haslam et al., 2013). One advance in increasing the accumulation of omega-3 LC-PUFAs in transgenic plants has derived from characterization of acyl CoA-dependent desaturases (Sayanova et al., 2011a), which bypass the well-documented substrate-dichotomy bottleneck (a result of the differing acyl carrier substrate preferences of desaturases and elongases; Abbadi et al., 2004). The benefits of using an acyl CoA-dependent Δ6-desaturase, such as that found in Ostrococcus tauri (Domergue et al., 2005), include not only the possibility of elevated EPA accumulation, but also a route to circumvent unwanted C18 biosynthetic intermediates (Sayanova et al., 2011a; Ruiz-Lopez et al., 2013). Accumulation of these intermediates (usually omega-6 acids) is characteristically associated with expression of phospholipid- dependent Δ6-desaturases (Abbadi et al., 2004; Sayanova et al., 2011a; Ruiz-Lopez et al., 2013) and is not seen in fish oil. Indeed, previous attempts to produce EPA have resulted in massive co-accumulation of the C18 omega-6 γ-linolenic acid (18:3 n-6, GLA) (Cheng et al., 2010).
Here, we demonstrate how a crop (C. sativa) may be successfully engineered to accumulate high levels of EPA and/or DHA in its seed oil. The choice of C. sativa as a host for the biosynthesis of omega-3 LC-PUFAs enables the introduced enzymes to use the endogenously accumulating high levels of a-linolenic acid (18:3 n-3, ALA), the starting substrate for the omega-3 biosynthetic pathway (Figure 1a). Furthermore, the high yield of target fatty acids was achieved without unwanted accumulation of intermediates and/or omega-6 PUFAs in the seed oil, and, most importantly, with efficient channelling of EPA and DHA into seed triacylglycerols.
Results and Discussion
Production of EPA and DHA in an oil seed crop
Two constructs encoding the primary biosynthetic activities for omega-3 LC-PUFA biosynthesis (p5_EPA and p7_DHA) were prepared for introduction into C. sativa. Construct p5_EPA was designed to produce high levels of EPA in seeds and contained five genes (Figure 1b), while construct p7_DHA was designed to accumulate both EPA and DHA, and contained seven genes (Figure 1b). In both cases, individual biosynthetic activities were encoded by codon-optimized sequences under the independent control of seed-specific promoters (Figure 1b; see also Experimental procedures). These two constructs were introduced into C. sativa plants via Agrobacterium-mediated floral transformation. The transformation of C. sativa and expression of these constructs resulted in no change in the germination, development or stature of the transgenic plants (Figure S1). Analysis of total fatty acid methyl esters (FAMEs) from the seeds of eight individual transgenic C. sativa T2 lines expressing the p5_EPA construct demonstrated accumulation of significant levels of EPA (Table S1). The mean levels of EPA found in these T2 lines ranged from 6.8 to 18.7 per cent of total fatty acids in mature seeds (mean 13.8 per cent). As observed previously, the amount of oleic acid (18:1 n-9, OA) decreased dramatically from 14.5 per cent in the untransformed control to 5.9 per cent in transgenic C. sativa seeds expressing the O. tauri D6-desaturase (Sayanova et al., 2011a). In agreement with previous results obtained with expression of acyl CoA D6-desaturases in transgenic plants (Hoffmann et al., 2008; Petrie et al., 2010; Sayanova et al., 2011a; Ruiz-Lopez et al., 2012, 2013), only a minor accumulation of C18 D6-desaturated fatty acids was observed: the mean levels of GLA and stearidonic acid (18:4 n-3, SDA) in T2 seeds were 2.7 per cent and 2.9 per cent of total fatty acids, respectively (Table S1). Similarly, the mean levels of the omega-6 LC-PUFA intermediates arachidonic acid (20:4 n-6, ARA) and dihomo-c-linolenic acid (20:3 n-6, DGLA) were 1.7 per cent and 0.8 per cent, respectively. Overall, levels of newly synthesized omega-3 fatty acids were much higher than those of omega-6 acids, with an n-3/n-6 ratio of 9:1. It is interesting to note that the health-promoting effects of LC-PUFAs are dependent on maintenance of the correct balance between omega-3 and omega-6 PUFA. A lower ratio of omega-6/omega-3 fatty acids is more desirable to reduce the risk of many of the chronic diseases of now prevalent in Western societies.
Analysis of seed FAMEs in control (Figure 2a) and T3 lines expressing p5_EPA showed an increase in EPA content (14.7 per cent of total fatty acids in seeds). In contrast to the mean T2 value, the highest EPA value observed in an individual T3 line (named RRes_EPA) was 23.9 per cent (Figure 2c and Table S1). Further analyses demonstrated that the highest EPA value observed in an individual seed of RRes_EPA was 31 per cent of total fatty acids (Figure 2d). Previous reports on high EPA accumulations (e.g. Cheng et al., 2010) focused on the zero-erucic acid Brassica carinata, with a mean EPA level of 20.4 per cent in transgenic seeds. However, the potential of B. carinata as a source of omega-3 was compromised by the inefficient D6-elongation step, which resulted in accumulation of GLA and SDA (26.9 per cent and 5.4 per cent of total fatty acids, respectively).
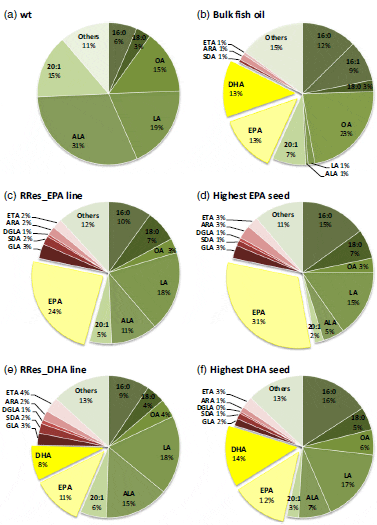
After achieving a significant yield of EPA, the next goal was to produce both EPA and DHA in C. sativa seeds to levels similar to those in fish oil (Figure 2b). Engineering a metabolic route to DHA concomitant with EPA has been challenging, with many efforts resulting in some accumulation of EPA and docosapentaenoic acid (22:5 n-3; DPA), although characteristically only small DHA accumulations were recorded. We and others have shown how DHA may be accumulated in the seed oil of the model Arabidopsis, with accumulation of up to 15 per cent DHA in Arabidopsis seed oil (Petrie et al., 2012; Ruiz-Lopez et al., 2013). However, levels of EPA in this line were extremely low (1.8 per cent; Petrie et al., 2012). Combining our knowledge of how to produce significant accumulations of EPA with our previous experience of DHA synthesis in Arabidopsis (Ruiz-Lopez et al., 2012, 2013), we identified a transgene combination (p7_DHA) to successfully direct accumulation of significant levels of EPA and DHA in C. sativa. The mean yields of EPA and DHA in C. sativa T2 lines expressing p7_DHA were 6.2 per cent and 5.2 per cent of total fatty acids in seeds (Table S1). Moreover, as was the case with RRes_EPA, these lines showed low accumulation of C18 Δ6-desaturated fatty acids and ARA. The line accumulating the highest amount of DHA (named RRes_DHA) showed a mean accumulation of 11 per cent EPA and 8 per cent DHA in its seeds (Figure 2e). In addition, further single-seed analysis of the RRes_DHA line revealed individual seeds that accumulated up to 12 per cent EPA and 14 per cent DHA (Figure 2f), values very similar to those found in fish oil (mean of 13 per cent EPA and 13 per cent DHA, Figure 2b), but completely absent from wild-type C. sativa (Figure 2a). The accumulation of EPA and DHA was achieved with only minimal synthesis of intermediates (Figure 2, shown in shades of red), thus avoiding problems in human fatty acid metabolism or interference with the benefits of omega-3 fatty acids.
Lipid profiling of omega-3-accumulating lines
To further characterize the accumulation of LC-PUFAs in transgenic C. sativa, a more detailed lipid class analysis was performed on seeds of both RRes_EPA and RRes_DHA lines. Analyses of neutral and polar lipids from the seed oil of RRes_EPA revealed that EPA was almost equally distributed between neutral lipids (19 per cent) and polar lipids (17.1 per cent; see Table 1). This pattern of partitioning is slightly reversed compared with our previous observations in Arabidopsis seeds expressing a similar EPA construct, where EPA comprised 8.7 per cent of total fatty acids in neutral lipids and 11.5 per cent in polar lipids (Ruiz-Lopez et al., 2013). Characterization of seed lipids from RRes_DHA clearly showed accumulation of DHA in neutral lipids, although polar lipids showed a higher accumulation of DHA (8.5 per cent versus 4.5 per cent; Table 1).
To better understand the distribution of these non-native fatty acids in C. sativa seeds, we performed a more detailed examination of the lipidome, using similar approaches as previously described for Arabidopsis (Ruiz-Lopez et al., 2013). First, the distribution of EPA and DHA in neutral lipids was determined for the RRes_EPA and RRes_DHA lines, compared with wild-type C. sativa (Table 2). Interestingly, this revealed a difference in the accumulation of EPA and DHA into triacylglycerols (TAG), as EPA levels were greater in TAG than diacylglycerols (DAG) for RRes_EPA, whereas the reverse was true for DHA in RRes_DHA.
To develop a more accurate hypothesis regarding the flux of these fatty acids through various lipid classes, we also determined the acyl composition of the major phospholipids phosphatidylcholine (PC), phosphatidylethanolaomine (PE), phosphatidylinositol (PI) and phosphatidylserine (PS) (Table 3 and Figure S2A–F) in order to evaluate the PC-DAG-TAG model proposed by Bates and Browse (2012), in which extraplastidial fatty acids accumulate in triacylglycerol via PC and then DAG, rather than direct acylation into neutral lipids from the acyl CoA pool. The fatty acid composition of PC, PE and PI+PS was determined for RRes_EPA, RRes_DHA and wild-type C. sativa mature seeds (Table 3 and Figure S2A–F), revealing a precise pattern of distribution for non-native omega-3 LC-PUFAs among these phospholipids.
In the case of EPA in RRes_EPA, there was a very similar level of accumulation (approximately 15 per cent) in all phospholipids, whereas in RRes_DHA, the accumulation of DHA was slightly biased towards PE, with little accumulation in PI+PS (Table 3). Such a pattern of accumulation of EPA and DHA is similar to that observed in transgenic Arabidopsis seeds accumulating these fatty acids (Ruiz-Lopez et al., 2013). Examination of the accumulation of EPA and DHA across PC, DAG and TAG (Tables 2 and 3) revealed discrete patterns for retention or accumulation of these two fatty acids.
In the case of EPA, accumulation follows the pattern TAG > DAG > PC (21.1 per cent; 17.8 per cent: 15.6 per cent; Tables 2 and 3), consistent with the Bates and Browse (2012) model of flux through PC. Similar patterns of accumulation are observed for endogenous fatty acids such as ALA and 20:1. In the case of DHA, the accumulation pattern is reversed to PC > DAG > TAG (7.3 per cent; 5.9 per cent; 4.5 per cent; Tables 2 and 3), indicating inefficient flux of this C22 LC-PUFA compared with the C20 form. Similar PC-biased accumulation of DHA was previously observed in Arabidopsis (Ruiz-Lopez et al., 2013), although a similar pattern was also observed for EPA in that species. However, in the present case of C. sativa, it is clear that, while EPA accumulates in TAG via DAG (presumably derived from PC, rather than via de novo synthesis, according to the model proposed by Bates and Browse, 2012), this is not the case for DHA. Clearly, TAG synthesis is an aggregation of a number of distinct pathways, and, in transgenic C. sativa, there appears to also be some acyl exchange between the glycerolipid pool and the acyl CoA pool, as both EPA and DHA are present in the latter (Table 4). In the case of DHA, this may represent an alternative route into TAG.
As well as the accumulation patterns for EPA and DHA described above, there are a number of other alterations to the lipid composition of transgenic C. sativa accumulating EPA and DHA, the most notable of which is a very pronounced decrease in the accumulation of oleic acid (OA; 18:1D9). This is true for both EPA- and DHA-accumulating lines, and was observed in neutral lipids (Table 2), phospholipids (Table 3) and acyl CoAs (Table 4). One interpretation for this inverse relationship with omega-3 LC-PUFA accumulation is that OA acts as a primary substrate for the synthesis of EPA and DHA, with distinct pools of this fatty acid (most likely in DAG) being channelled towards their synthesis. The fact that accumulation of endogenous metabolites of OA such as linoleic acid (LA; 18:2Δ912) (desaturation) or 20:1 (elongation) is not so dramatically altered in the transgenic lines compared with the wild-type may be interpreted as supporting such a scenario. Interestingly, and again highlighting the difference between C. sativa and Arabidopsis, the most dramatically alteration in fatty acid level in transgenic omega-3 LC-PUFA-accumulating Arabidopsis lines was a decrease in LA, which was observed in both neutral and phospholipid fractions (Ruiz-Lopez et al., 2013). Thus, while our data suggest the importance of a PC-derived DAG pool in TAG synthesis in C. sativa, analogous to Arabidopsis, they also suggest some significant features that make C. sativa a superior host for accumulation of EPA and DHA, and also question the assertion that Arabidopsis is a useful predictive model for events in C. sativa. However, further studies are required to better address this latter point.
To further define the acyl acylation of EPA and DHA into seed oil, and also possible biosynthetic fluxes, region-specific analyses were performed on RRes_EPA and RRes_DHA in order to determine the position on the glycerol backbone of EPA and DHA in both TAG and PC molecules. This analysis demonstrated the inherent bias of C. sativa acyltransferases with regard to positioning EPA and DHA at the sn-1/sn-3 positions in seed TAG (Figure 4). This was also true for eicosatetraenoic acid (20:4 n-3; ETA) and endogenous 20:1, whereas some endogenous fatty acids (such as OA, LA and to a lesser extent ALA) were biased towards the sn-2 position of TAG (Figure 4). The positional distribution of EPA and DHA showed a threefold enrichment at the sn-1/sn-3 position compared with the sn-2 position. Similarly, the distribution of EPA and DHA in PC was biased towards the sn-2 position, confirming the critical role of sn-2 PC in the biosynthesis (D5 and D4-desaturation) of omega-3 LC-PUFAs. Therefore, EPA and DHA are ultimately synthesized on sn-2 PC, and then channelled from this lipid to the sn-3 position of TAG, although whether this occurs directly via acyl CoA-independent pathways or via acyl exchange into the acyl CoA pool and subsequent acyl CoA-dependent acylation is unclear. As discussed above, the role of DAG as an intermediate may be inferred, at least in the case of EPA, although it remains to be determined if this occurs by incorporation into de novo DAG or via head group exchange with PC.
However, our studies confirm the crucial role that the endogenous seed lipid metabolism of the host plant plays in successful reconstitution of this heterologous pathway. Given that this varies from species to species, a clear understanding of the biochemical context into which transgenic metabolic pathways are inserted is essential, as recommended by Bates and Browse (2012) in their discussion of the accumulation of unusual C18 fatty acids.
Characterization of omega-3 in TAG molecular species
To better understand the accumulation of the target fatty acids within TAG, we performed LC-MS/MS analyses to identify the individual molecular species of TAG containing EPA and/or DHA present in mature seed oils of transgenic RRes_EPA and RRes_DHA T3 lines. As a result, a diverse collection of EPA- and DHA-containing TAG was identified (Figure S3A–C). In the RRes_EPA line (Figure S3A), the predominant EPA-containing TAG comprised TAG species containing a single EPA chain. The most abundant TAG species were EPA–OA–LA, EPA–ALA–20:1, EPA–18:0–20:1 and EPA–20:0–20:3, with mean levels of 11.5 per cent, 10.0 per cent, 11.0 per cent and 8.7 per cent of total EPA-containing TAG, respectively. Four TAG species in the RRes_EPA line comprised two EPA and one of the following fatty acids: LA, 20:0, 20:1 or ARA (13.0 per cent of total EPA TAG). We also detected a tri-EPA TAG (1.5 per cent of total EPA TAG). The capacity of C. sativa TAG to accommodate incorporation of more EPA (and DHA) was examined by parallel FAME analyses of each sample (Figure S4A); the data clearly demonstrate how the contribution of TAG species containing two or more EPA increased proportionately with the percentage total EPA content. Furthermore, parallel FAME analysis of RRes_DHA TAG (Figure S4B) showed that concomitant accumulation of EPA and DHA is not restricted by TAG biosynthesis in C. sativa seed oil.
Targeted TAG LC-MS/MS analysis of the RRes_DHA line (Figure S3B,C) identified the major DHA-containing TAG. These were DHA–LA–ALA (mean 14.5 per cent), DHA–LA–LA (13.8 per cent) and DHA–ALA–ALA (11.3 per cent), which are notably different in their composition of endogenous fatty acids compared with TAG from RRes_EPA (above). We also observed appreciable levels of TAG containing two DHA molecules (6.8 per cent of total DHA-containing TAG) and very low levels of tri-DHA TAG (approximately 0.3 per cent of total DHA TAG). Both EPA and DHA were also found in the same TAG species (Figure S5), e.g. in combination with OA and 18:0. There was also a modification of the pattern of EPA accumulation in the TAG species of the RRes_DHA line. In order to accommodate the synthesis and storage of DHA, single molecules of EPA were distributed across a wider variety of TAG species, rather than accumulating in the four specific TAG seen in RRes_EPA. Collectively, these observations on the accumulation of EPA or DHA confirm the disparity in the channelling of these two non-native fatty acids, and indicate the need for further studies to better define their path of deposition in TAG.
Different plants probably utilize a combination of routes to incorporate novel fatty acids into TAG (Bates et al., 2013). However, it is clear that PC plays a central role, with newly synthesized LC-PUFAs, such as EPA and DHA, under a constant dynamic exchange with the acyl CoA pool in a process described as acyl editing. Removal of an LC-PUFA from PC may proceed by the reverse action of acyl CoA: lysophosphatidylcholine acyltransferase or the combined action of phospholipase A2 and long-chain acyl CoA synthetase. Once in the acyl CoA pool, EPA-CoA and DHA-CoA and glycerol-3-phosphate may be converted into TAG in a series of reactions known as the Kennedy pathway (Bates and Browse, 2012). Alternatively, the PC head group may be removed, producing a DAG molecule containing EPA or DHA at the sn-2 position. This reaction may proceed via four enzymatic mechanisms: phospholipase C, phospholipase D together with phosphatidic acid phosphatase, the reverse action of CDP-choline:diacylglycerol cholinephosphotransferase, or the recently identified phosphatidylcholine: diacyglycerol cholinephosphotransferase. The DAG produced by these mechanisms may then be utilized to produce TAG. Furthermore, direct transfer of the sn-2 EPA or DHA of PC to the sn-3 position of DAG is possible, generating TAG via a phospholipid:diacylglycerol acyltransferase. The analysis of C. sativa seed oil has demonstrated the capacity of this plant to accumulate high levels of novel fatty acids in TAG, and suggests that there may be specific lipid pools, separate to those for membrane synthesis, that channel EPA and/or DHA into TAG. How this may be achieved remains unclear: it may involve a subset of specific enzymes or a spatial separation in the ER membrane (see Chapman and Ohlrogge, 2012); however, it is clear that manipulation of plant seed oil composition is heavily dependent on the metabolic pathways of the host.
In conclusion, the accumulation of high levels of EPA and/or DHA in plant oils is an important goal of the metabolic engineering community. In this study, we have successfully reconstituted the EPA and DHA biosynthetic pathway in the seeds of an oilseed crop, C. sativa, demonstrating not only the practical feasibility of large-scale production of these important omega-3 fatty acids in an oilseed crop at comparable levels to those found in fish oils, but also a composition that matches that of marine sources.
February 2014




