Many feed ingredients used in aquaculture have been found to be frequently contaminated (Cagauan et al., 2004). At the present time, increased use of ingredients of plant origin in aquafeed formulations for fish culture has intensified the potential onset for aflatoxicosis in farming systems due to the carryover of high loads of aflatoxin contamination by vegetable sources (Murjani, 2003).
Aflatoxin B1 (AFB1) is produced by Aspergillus species on agricultural commodities (Leontopoulos et al., 2003). AFB1 (C17 H12 O6) is an abundant and toxic mycotoxin, where animals that consume aflatoxins (AFs) contaminated feed develop various health problems including growth retardation, reduction of feed efficiency and liver and kidney damage (Bintvihok, 2002). AFs are known as a hepatocarcinogens in various animal species, including fish (Allameh et al., 2005). It is also a suspect human carcinogen and has been shown to play a role in human hepatocarcinoma (Wang et al., 2001).
Liver is the target organ of AFs and hepatobiliary damages are associated with pathological changes and alterations in liver function enzymes (Sur and Celik, 2005). Moreover, Santacroce et al. (2008) reported that AFB1 is the most biologically active toxin known and has been found to produce hepatotoxic, carcinogenic, mutagenic, teratogenic and immunosuppressant effects in aquatic animals.
Herbal medicinal products are widely used around the world (Jordan et al., 2010). A growing interest has emerged in using herbs in animal feeds by both researchers and feed companies (Logambal and Michael, 2000). The herbal biomedicinal active principles in the aquaculture antimicrobial capability and antistress characteristics due to the active principle natures such as alkaloids, flavanoids, pigments, phenolics, terpenoids, starch, steroids and essential oils. This practice will reduce the side effects of applying the synthetic compounds and the cost and also make it eco-friendly (Cowan, 1999). Hence, the alternative herbal biomedicines prove to be very effective in aquacultural operations (Citarasu, 2010). So, extracts from many oriental spice plants and herbs such as cinnamon, clove, garlic, sage, oregano, thyme, rosemary, mint, and vanilla have been known to possess antimicrobial effects (Tassou et al., 2000; Yildirim et al., 2000 and Abdelhamid et al., 2002 c).
Aflatoxicosis in farm animals is underestimated because of the lack of specific clinical signs that would be useful to establish an early diagnosis. Information regarding the impact of AFs on farm animals is mostly available for mammalian and terrestrial vertebrates. In contrast, there are very limited data on aquacultured species, thus the lack of information regarding the incidence of aflatoxicosis in farm-reared aquatic species may be in part due to the difficulty in accurately diagnosing aflatoxicosis in fish (Santacroce et al., 2008). So, the present work was designed to evaluate the ability of some medicinal herbs namely black seed, liquorices, onion, garlic, fenugreek and cinnamon ceylon (at a level of 1 per cent) against dietary AFB1 (150 ppb) effects on clinical lesions, postmortem examination and histopathological alterations in liver of mono-sex Nile tilapia (O. niloticus) fingerlings for 15 week.
Materials and Methods
The present work was carried out at the Department of Animal Production, Faculty of Agriculture, Kafr EL-Sheikh University, Egypt. A basal diet (28.66 per cent crude protein, 18.82 per cent ether extract, 7.51 per cent crude fiber, 6.44 per cent ash, 38.57 per cent nitrogen free extract, 496.93 Kcal / 100g DM gross energy (GE) and 57.67 mg CP / Kcal GE, P / E ratio), where GE (Kcal / 100 g DM) = CP x 5.64 + EE x 9.44 + Total carbohydrates x 4.11 (Macdonald et al., 1973). The diet was formulated from the commercial ingredients (fish meal 10 per cent, soybean meal 40 per cent, yellow corn 34 per cent, wheat bran 11.5 per cent, sunflower oil 4 per cent and vit. & min. mixture 0.5 per cent). The ingredients and supplemented medicinal herbs were bought from the local market. These ingredients were milled and AFB1 at level of 150 ppb besides the tested medicinal herbs (as an anti-toxin, were added at a level of 1 per cent) were mixed well all together and pressed by manufacturing machine (pellets size 1mm).
The experimental groups are described in Table (1). AFB1 was produced through pellets fermentation using toxigenic fungal strain of Aspergillus parasiticus NRRL 2999 (which was obtained from Mycotoxins Lab., National Research Center, Cairo, Egypt). Identification of AFB1 was done by thin layer chromatography and spectrophotometric [Spectronic® 20+ series spectrophotometers, Spectronic Unicam, 820 Linden Avenue, Rochester, NY 14625, USA] and quantification according to Karunaratne et al. (1990). Concentration of the produced AFB1 was calculated and incorporated into the experimental diets at a level of 150 ppb.
Table 1: Description of the experimental groups
A group of 360 mono-sex Niletilapia O. niloticus fingerlings with an average initial body weight of 10 ± 0.38 g was used in this study. Fish were obtained from a private hatchery in Al-Reiad, Kafr El-Sheikh governorate, Egypt and transported to the wet lab., Faculty of Agriculture, Kafr El-Sheikh University, Egypt. They were maintained in aquaria for one month before the beginning of the experiment for acclimatization purpose. The fish were fed during the acclimatization period on artificial basal diet at a rate of 3 per cent of the body weight. Mono-sex O. niloticus were randomly distributed into aquaria at a stocking rate of 15 fish per aquarium. Fish were assigned into three aquaria for each treatment (as replicates). Water of each glass aquarium (60 × 35 × 40 cm) was continuously supplied with a compressed air from an electric compressor. Dechlorinated tap water was used to change one third of the water in each aquarium daily.
During the experimental period (15 week), the fish were fed the experimental diets at a rate of 3 per cent of the live body weight daily. The diet was introduced twice daily, at 8.0 a.m. and 2.0 p.m. The amount of feed was adjusted weekly based on the actual body weight changes. Samples of water were taken daily before and after adding the diets from each aquarium to determine water quality parameters. Since, water temperature degree ranged between 23.0 and 25.0°C, pH values 6.5 – 8.0 and dissolved oxygen 6.0 – 7.0 mg / L, which were suitable for rearing O. niloticus according to Lim and Webster (2006). Light was controlled by a timer to provide a 14h light: 10h dark as a daily photoperiod.
Through all the intervals of the experimental period, the clinical signs and postmortem examination of the aflatoxicated fish were recorded by a digital camera, CASIO, Exilim optical 3x, 6.0 Mega pixels, 2.5" LCD, Anti-shake DSP, CASIO Computer Co., LTD., Tokyo, Japan. Also, at the end of the experiment, three fishes from each treatment were sacrificed and the target organ (liver) was sampled. Samples were fixed in 10 per cent neutralized formalin solution followed by washing with tap water, then dehydrated by different grades of alcohol (70 per cent, 85 per cent, 96 per cent and 99 per cent). Samples were cleared by xylene and embedded in paraffin wax. The wax blocks were sectioned to six microns. The sections were stained by hematoxylin (H) and eosin (E) and then subjected to a microscopic examination according to Roberts (2001).
Results and Discussion
Clinical signs and postmortem examination:





Through all the interval periods of the present study, the clinical signs and postmortem lesions of the aflatoxicated fish (G2 – G8) were recorded. These signs included protrusive eye (Fig. 4), slow-motion, lethargy, less feed acceptability, skin covered by thick mucus, severe hemorrhage on dorsal, anal and caudal fins, discarded scales and caudal fin erosion and loss construction of the body of aflatoxicated fish (Fig. 2), as compared to a normal appearance of the fish in the control group (G1, Fig. 1).
Anatomically, there were some postmortem lesions included uncharacterized liver, viscera of fish and the abdominal cavity were filled with heavy amounts of mucus, damaged liver and enlargement of gall bladder (which was filled with bile) of aflatoxicated fish (Figs. 4 & 5). While, the liver of fish fed the control diet (G1) appeared with normal structure (Fig. 3).
Similar findings were recorded by Hussein et al. (2000); Abdelhamid et al. (2002 a & b) and Tuan et al. (2002). In addition, evidence of morphological alterations in O. niloticus fed aflatoxin-contaminated feeds include ocular opacities leading to cataracts and blindness; lesions on the body surface such as fin and tail rot; yellowing of the body surface, termed "yellowing tilapia"; abnormal swimming patterns and anorexia were recorded also by Cagauan et al. (2004).
Similarly, Mehrim et al. (2006) observed the same external symptoms and postmortem signs of aflatoxicated O. niloticus. Moreover, El-Barbary and Mehrim (2009) confirmed that the most common clinical sings observed on aflatoxicated O. niloticus were lethargy, loss of appetite, sluggish movement, dark discoloration of the skin and respiratory manifestations. Also, they added that the common lesions noticed by necropsy of aflatoxicated fish were accumulation of fluid in abdomen, congested gills and dark liver.
Generally, it is difficult to recognise or diagnose this condition because of its slow, subclinical trend. The majority of clinical signs is related to chronic, impaired liver function, such as reduced feed efficiency, weight loss, increased susceptibility to secondary infectious diseases, necrosis and tumor development in the liver and other organs, and increased mortality (Santacroce et al., 2008).
Histopathological changes:
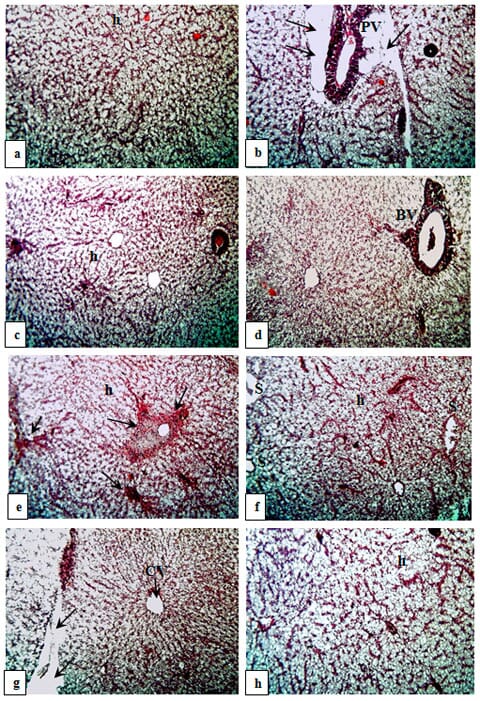
Liver is the most susceptible organ to AFB1 injury in fishes (Hendricks, 1994). The present histological findings in liver of O. niloticus fed BR free from AFB1 (G1) or aflatoxicated diets without (G2) or with tested medicine herbs (G3 – G8) are shown in Figures 6 a – h.
AFB1 is hepatotoxic and hepatocarcinogenic in several animal species at a very low concentration (Sharma et al., 2011). Hence, the histological examination in the present study indicated that AFB1 induced severe histological changes in the hepatic tissues. Similar histological changes in the liver of O. niloticus have been documented previously (Abdelhamid et al., 2004 and Mehrim et al., 2006).
In addition, El-Barbary and Mehrim (2009) found that AFB1 presented histopathological changes in hepatopancrease of O. niloticus, which increased in severity with increasing AFB1 level. Furthermore, Deng et al. (2010) reported that the liver of tilapia fed with 638 and 793 μg AFB1 / kg diet were infiltrated by many inflammatory cells and eosinophilic materials. In this respect, the pathological changes of liver observed in the present investigation may be due to primary site of metabolism of ingested aflatoxins as well as the primary laceratian of residues and lesions (Newperne, 1999).
Histopathological alterations like necrosis, fibrosis and lymphocytolysis in liver and other organs had been observed due to aflatoxin exposure in different fish species such Labeo rohita (Mohapatra et al., 2011); Clarious lazara (Zaki et al., 2002); channel catfish (Jantrarotai and Lovell, 1990) and hybrid sturgeon Acipenser ruthenus ? x A. baeri ? (Raghavan et al., 2011). In contrary, no significant histological lesions were identified in the liver of gibel carp (Carassius auratus gibelio) treated with AFB1 (Huang et al., 2011), as well as they indicated that gibel carp is a less susceptible species to AFB1 exposure up to approximately 1000 ppb, at least for 12 weeks.
Furthermore, experimental studies on various species of rodents, birds and fishes indicated that aflatoxins are capable to induce oxidative damage in the cells and produce reactive oxygen species (ROS), such as superoxide radicals, hydroxyl radicals and hydrogen peroxides in hepatocytes (Abdel-Wahhab et al., 2007 & 2010). Additionally, Alpsoy and Yalvac (2011) reported that elevation of ROS levels causes inhibitory effects on biological processes including DNA synthesis, DNA-dependent RNA synthesis, DNA repair, and protein synthesis. Thus, the extent of the damage produced by aflatoxins depends on the toxin concentration present in foods or feeds and also the period of exposure, as well as animal species susceptibility (Stewart and Larson, 2002).
Because of herbal products or medicinal herbs are cheaper source for therapeutics, they have greater accuracy than chemotherapeutic agents, and offer a viable solution for all problems which aquaculture faces today (Citarasu et al., 2002, 2003 a & b); as well as they contain potentially active antioxidant and antimicrobiological principles (Sivaram et al., 2004); antifungal, antiaflatoxigenic and antioxidant compounds (Kumar et al., 2007); so, currently histological findings revealed the positive effects of tested medicine herbs, which alleviated the hepatotoxic effects of AFB1 on the experimental fish. Similarly, numerous scientific efforts were conducted for using medicine herbs against toxic effects of AFB1 (Madhusudhanan et al., 2006; Mehrim et al., 2006 and El-Barbary, 2008).
Yet, the potential of some herbal plants to degrade AFs has been detected by Sandosskumar et al. (2007), whereas garlic (Allium sativum L.) and onion (A. cepa L.) roots incubated in water containing 70 mg of AFB1 for 5 days cause 58.5 per cent reduction in aflatoxin level. Also, the impact of both of medicinal herbs, namely rosemary and parsley, at a low level (2 g / kgB.W.) to eliminate the drastic effects of AFB1 on O. niloticus were reported by El-Barbary and Mehrim (2009).
Moreover, medicinal herbs contain a number of bioactive compounds e.g., glycyrrhizin and its aglycon glycyrrhetic acid, liquiritin, liquiritin apioside, isoliquiritin and glabridin, as well as contain several active components, such as polysaccharides, alkaloids and/or flavonoids (Cinatl et al., 2003). Hence, glycyrrhizin containing one molecule of glycyrretinic acid, which has anti-inflammatory and anti-tumor activities, mediated by its immunomodulatory activities (Zhang et al., 1990).
Yet, flavonoids are typical phenolic compounds has long been recognized to possess anti-inflammatory, antioxidant, antiallergic, hepatoprotective, antithrombotic, antiviral, anticarcinogenic activities and free radical scavengers (Carroll et al., 1998), as well as, Citarasu et al. (1998) found that herbal compounds have the ability to inhibit the generation of oxygen anions and to scavenge free radicals. So, the alternative herbal biomedicines prove to be very effective in aquacultural operations (Citarasu, 2010).
Consequently, administration of ginseng and Nigella sativa oil via injection 1 per cent of body weight reduced the development of hepatotoxicity by AFB1 and may protect liver from free radical reactions due to aflatoxin (Zaki et al., 2011). More recently, El-Nagerabi et al. (2012) found that calyx of Hibiscus sabdariffa and Nigella sativa oil can be effectively used as biologically safe control additives against aflatoxin contamination of human food, animal feed and other agricultural products. Additionally, liver detoxification function it self was discussed by Apte and Krishnamurthy (2011), where detoxification function of the liver is highly complex involving hundreds of different enzymes with unique substrate specificity, regulation, and activity.
Conclusions
Based on the foregoing results, hepatotoxic effects of AFB1 were indicated on mono-sex O. niloticus fingerlings. Also, it is of interest to conclude the safety and useful addition of tested medicine herbs to eliminate the toxic effects of AFB1, especially 1 per cent black seed (Nigella sativa, G3) and/or ceylon cinnamon (Cinnamomum verum, G8). Furthermore, Kumar et al. (2011) reported that a combination of different herbal extracts/'fractions is likely to provide desired activities to cure severe liver diseases. Development of such medicines with standards of safety and efficacy can revitalise treatment of liver disorders and hepatoprotective activity. So, further studies are needed for studying the effect of the combination of these medicine herbs against the toxic effects of various AFB1 levels or other mycotoxins on tilapia or other fish species at different ages, physiological stages or nutritional status.
August 2013




