Ammonia is toxic to fish if allowed to accumulate in fish production systems. When ammonia accumulates to toxic levels, fish can not extract energy from feed efficiently. If the ammonia concentration gets high enough, the fish will become lethargic and eventually fall into a coma and die.
In properly managed fish ponds, ammonia seldom accumulates to lethal concentrations. However, ammonia can have so-called “sublethal” effects—such as reduced growth, poor feed conversion, and reduced disease resistance—at concentrations that are lower than lethal concentrations.
Effects of pH and temperature on ammonia toxicity
Ammonia in water is either un-ionised ammonia (NH3) or the ammonium ion (NH4 +). The techniques used to measure ammonia provide a value that is the sum of both forms. The value is reported as “total ammonia” or simply “ammonia.” (In this publication, “ammonia” refers to the sum of both forms; the specific forms will be referred to as appropriate.) The relative proportion of the two forms present in water is mainly affected by pH. Un-ionised ammonia is the toxic form and predominates when pH is high. Ammonium ion is relatively nontoxic and predominates when pH is low. In general, less than 10 percent of ammonia is in the toxic form when pH is less than 8.0. However, this proportion increases dramatically as pH increases (Fig 1).
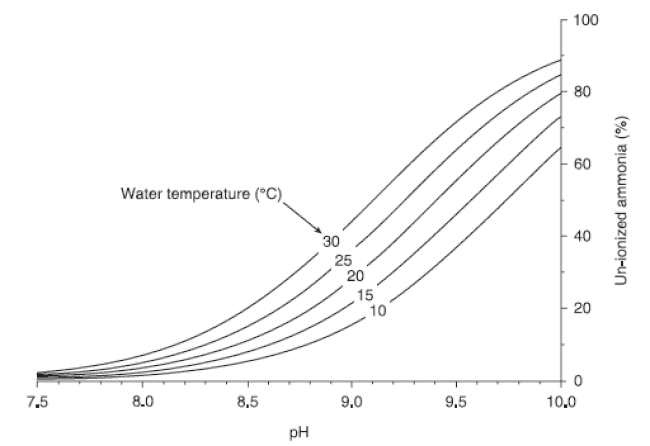
To determine the proportion of un-ionised ammonia in a water sample, draw a line from the pH of the water straight up to the line that is closest to the water temperature. From that point, draw a line to the right until it intersects the graph’s vertical axis. That point is an estimate of the percentage of un-ionised ammonia in the water sample. Now, simply multiply that number (divided by 100) by the total ammonia concentration to estimate the un-ionised ammonia concentration.
In ponds, pH fluctuates with the photosynthesis (which increases pH) and respiration (which reduces pH) of pond organisms. Therefore, the toxic form of ammonia predominates during the late afternoon and early evening (Fig 2) and ammonium predominates from before sunrise through early morning.
The equilibrium between NH3 and NH4 + is also affected by temperature. At any given pH, more toxic ammonia is present in warmer water than in cooler water (Fig 1).
Ammonia dynamics in fish ponds
The measurement of ammonia concentration (and that of many other water quality variables) provides only a snapshot of conditions at the time a water sample is collected. A single measurement provides no insight into the processes that affect ammonia concentrations; it is simply the net result of processes that produce ammonia and processes that remove or transform ammonia. The relationships among these processes are complex, but the important point is that the rates change differentially throughout the year and result in the measured patterns.
Ammonia sources
The main source of ammonia in fish ponds is fish excretion. The rate at which fish excrete ammonia is directly related to the feeding rate and the protein level in feed. As dietary protein is broken down in the body, some of the nitrogen is used to form protein (including muscle), some is used for energy, and some is excreted through the gills as ammonia. Thus, protein in feed is the ultimate source of most ammonia in ponds where fish are fed.
Another main source of ammonia in fish ponds is diffusion from the sediment. Large quantities of organic matter are produced by algae or added to ponds as feed. Faecal solids excreted by fish and dead algae settle to the pond bottom, where they decompose. The decomposition of this organic matter produces ammonia, which diffuses from the sediment into the water column.
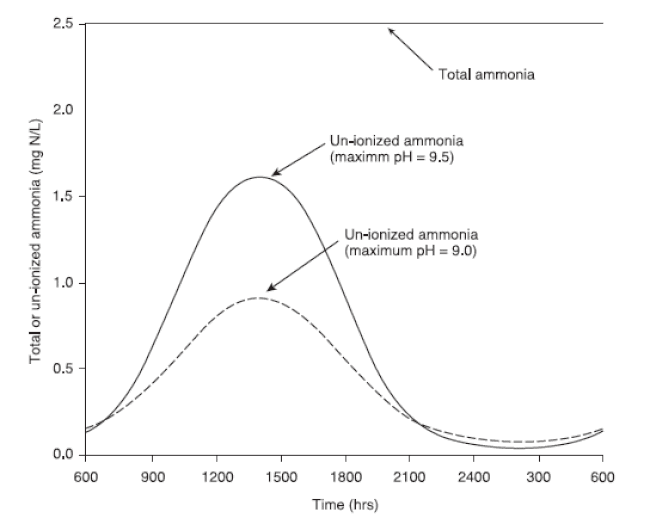
The top horizontal line indicates a total ammonia concentration of 2.5 mg N/L, which is assumed not to change during the day. The two curved lines indicate daily changes in un-ionised ammonia concentration where the maximum afternoon pH is 9.0 or 9.5. These conditions indicate that fish may be exposed to toxic, un-ionised ammonia concentrations for brief periods during the late afternoon.
Ammonia sinks
There are two main processes that result in the loss or transformation of ammonia. The most important is the uptake of ammonia by algae and other plants. Plants use the nitrogen as a nutrient for growth, “packaging” the nitrogen in an organic form. Algal photosynthesis acts like a “sponge” for ammonia, so anything that increases overall algal growth will increase ammonia uptake. Such factors include sufficient light, warm temperature, abundant nutrient supply, and (to a point) algal density.
The other important process of ammonia transformation in fish ponds is “nitrification.” Bacteria oxidise ammonia in a two-step process, first to nitrite (NO2 -) and then to nitrate (NO3 -). The main factors that affect nitrification rate are ammonia concentration, temperature and dissolved oxygen concentration. During summer, ammonia concentration is very low and so nitrification rates are also very low. During winter, low temperature suppresses microbial activity. During spring and fall, ammonia concentration and temperature are intermediate, conditions that favour maximum nitrification rates. Spring and fall peaks of nitrite concentration are commonly seen in fish ponds.
Other processes, such as the volatilisation of ammonia gas from the pond surface into the air, are responsible for a relatively small and variable amount of ammonia loss from fish ponds.
When is ammonia most likely to be a problem?
In fish ponds, it is extremely unlikely that un-ionised ammonia would accumulate to a concentration that would become toxic enough to kill fish. However, un-ionised ammonia will occasionally accumulate to levels that cause sub-lethal effects.
The following analysis is based on water quality criteria for ammonia developed by the US Environmental Protection Agency (EPA). The EPA has established three kinds of criteria (one acute and two chronic) for ammonia (expressed as nitrogen), based on the duration of exposure.
The acute criterion is a 1-hour average exposure concentration and is a function of pH. One chronic criterion is the 30-day average concentration and is a function of pH and temperature. The other chronic criterion is the highest 4-day average within the 30-day period and is calculated as 2.5 times the 30-day chronic criterion. The EPA criteria help determine when ammonia might be a problem.
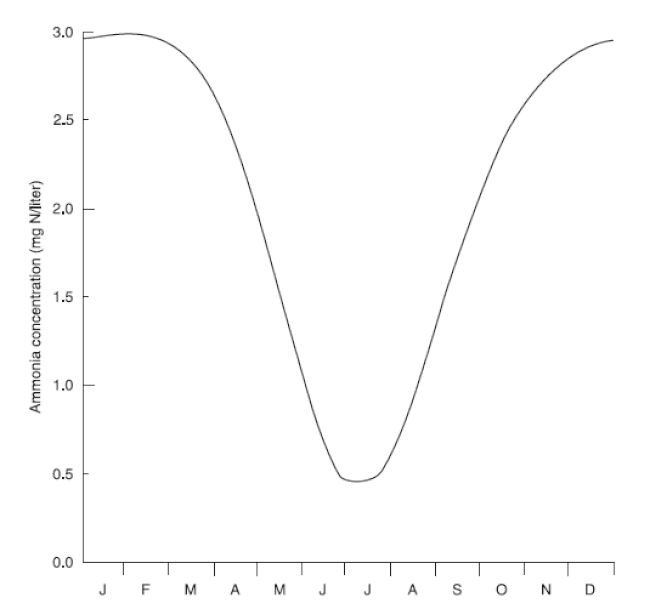
Ammonia concentration is generally lowest during summer and highest during winter.
During winter
It is generally assumed that ammonia is not a problem in the winter because feeding rates are very low. (Fish are fed on only the warmest days of winter, usually when the water temperature is higher than 50 °F.) However, ammonia concentration tends to be greater during winter (2.5 to 4.0 mg/L, or even higher) than during summer (less than 0.5 mg/L) (Fig 3).
The relatively low concentration during summer can be attributed to intense photosynthesis by algae, which removes ammonia. During winter, algae take up little ammonia but the ammonia supply continues, primarily from the decomposition of organic matter that accumulated on pond sediment during the growing season. In general, the magnitude and duration of high ammonia concentrations during the late fall and winter can be related to the total amount of feed added to a pond during the preceding growing season.
The 30-day chronic criterion for ammonia (as nitrogen) in winter ranges from about 1.5 to 3.0 mg/L, depending on pH. Ammonia concentrations during the winter usually exceed this criterion. This may cause stress in fish at a time of year when the fish immune system is suppressed because of low temperature.
After the crash of an algae bloom
Some ponds have very dense algae blooms dominated by one or two species. For reasons that are not well understood, these blooms are subject to spectacular collapse, often called a “crash,” where all the algae suddenly die. When this occurs, ammonia concentration increases rapidly because the main mechanism for ammonia removal— algal uptake—has been eliminated. Rapid decomposition of dead algae reduces the dissolved oxygen concentration and pH and increases ammonia and carbon dioxide concentrations. After the crash of an algae bloom, ammonia concentration can increase to 6 to 8 mg/L and the pH can decline to 7.8 to 8.0. The 4-day chronic criterion, the appropriate criterion to apply following the crash of an algae bloom, ranges from about 2.0 mg/L at pH 8.0 to about 3.0 mg/L at pH 7.8. Therefore, ammonia concentration after the crash of an algae bloom may exceed the 4-day chronic criterion.
Occasionally during the late afternoons in late summer or early fall
Seasonal variation in ammonia concentration depends on algal density and photosynthesis. When these are high, ammonia concentration is low. Daily variation in the concentration of toxic, un-ionised ammonia depends on changes in pH (from photosynthesis) and, to a much lesser extent, temperature (Fig 2). In the late summer or early fall, ammonia concentration begins to increase but daily changes in pH remain large. In these situations, fish may be exposed to ammonia concentrations that exceed the acute criterion for a few hours each day. If late afternoon pH is about 9.0, the acute criterion is about 1.5 to 2.0 mg/L total ammonia-nitrogen. Total ammonia-nitrogen concentrations during summer are typically less than 0.5 mg/L, so fish are unlikely to be stressed if the late afternoon pH is less than 9.0.
It is difficult to be more precise about the risk of ammonia toxicity because of deficiencies in the methodology used in research. Nearly all ammonia toxicity tests are conducted in systems that maintain a constant ammonia concentration. These conditions do not reflect the fluctuating concentrations of NH3 in ponds. Accordingly, one must be careful when applying research results to production situations. For example, in one study, growth of channel catfish exposed to a constant ammonia concentration of 0.52 mg/L NH3 was reduced by 50 percent relative to unexposed fish. However, brief (2- to 3-hour) daily exposure to 0.92 mg/L NH3 (such as might occur in ponds) did not affect growth and feed conversion ratio. The fact that many fish can acclimate to repeated exposure to high concentrations of un-ionised ammonia is a further complicating factor.
Ammonia management options
On rare occasions ammonia concentration becomes high enough to cause problems. What practical steps can be taken if this occurs? The short answer is—not much.
Theoretically, there are several ways to reduce ammonia concentration, but most approaches are impractical for the large ponds used in commercial aquaculture. Following is a discussion of some options, their practicality and their effectiveness.
Stop feeding or reduce feeding rate
The primary source of nearly all the ammonia in fish ponds is the protein in feed. When feed protein is completely broken down (metabolised), ammonia is produced within the fish and excreted through the gills into pond water. Therefore, it seems reasonable to conclude that ammonia levels in ponds can be controlled by manipulating feeding rate or feed protein level. This is true to some extent, but it depends on whether you want to control it over the short-run (days) or the long-run (weeks or months).
In the short-run, sharp reductions in feeding rate have little immediate effect on ammonia concentration. The ecological reason for this is based on the complex movement of large amounts of nitrogen from one of the many components of the pond ecosystem to another. In essence, trying to reduce ammonia levels by withholding feed can be compared with trying to stop a fully loaded freight train running at top speed—it can be done but it takes a long time.
Producers can reduce the risk over the long-run by adjusting both feeding rate and feed protein level. Limit feed to the amount that will be consumed. In mid-summer the maximum daily feeding rate should be 100 to 125 pounds per acre. By feeding conservatively, the potential for high ammonia in ponds and the risks associated with sub-lethal exposure (disease, poor feed conversion, slow growth) can be minimised.
Increase aeration
The toxic form of ammonia (NH3) is a dissolved gas, so some producers believe pond aeration is one way to get rid of ammonia because it accelerates the diffusion of ammonia gas from pond water to the air. However, research has demonstrated that aeration is ineffective at reducing ammonia concentration because the volume of water affected by aerators is quite small in comparison with the total pond volume and because the concentration of ammonia gas in water is typically fairly low (especially in the morning). Intensive aeration may actually increase ammonia concentration because it suspends pond sediments.
Add lime
It has long been thought that liming ponds decreases ammonia concentrations. In fact, using liming agents such as hydrated lime or quick lime could actually make a potentially bad situation much worse by causing an abrupt and large increase in pH. Increasing pH shifts ammonia toward the form that is toxic to fish. In addition, the calcium in lime can react with soluble phosphorus, removing it from water and making it unavailable to algae.
In ponds with similar algal density, daily fluctuations of pH in low-alkalinity pond waters are more extreme than those in waters of sufficient alkalinity (greater than 20 mg/L as CaCO3; see SRAC Publication No. 464). Therefore, liming can moderate extreme pH values, particularly those that occur during late afternoon when the fraction of total ammonia that is in the toxic form is highest. However, this technique is effective only in ponds with low alkalinity. Most fish ponds have sufficient alkalinity. Increasing the alkalinity above 20 mg/L as CaCO3 will not provide additional benefit. Furthermore, liming does not address the root causes of high ammonia concentration; it only shifts the distribution of ammonia from the toxic to the non-toxic form by moderating high pH in the afternoon.
Fertilise with phosphorus
Most of the ammonia excreted by fish is taken up by algae, so anything that increases algal growth will increase ammonia uptake. This fact is the basis for the idea of fertilising ponds with phosphorus fertiliser to reduce ammonia levels. However, under “normal” pond conditions, algae blooms in fish ponds are very dense and the rate of algae growth is limited by the availability of light, not nutrients such as phosphorus or nitrogen. Therefore, adding phosphorus does nothing to reduce ammonia concentration because algae are already growing as fast as possible under the prevailing conditions.
The highest ammonia concentrations in fish ponds occur after the crash of an algae bloom. Fertilisation, particularly with phosphorus, may accelerate the re-establishment of the bloom, but most ponds have plenty of dissolved phosphorus (and other nutrients) to support a bloom and do not need more.
Reduce pond depth
Algal growth (and therefore the rate of ammonia uptake by algae) in fish ponds is limited by the availability of light. Anything that increases light increases ammonia uptake. Theoretically, dense algae blooms in shallow ponds will remove ammonia more effectively than the same dense blooms in deeper ponds. On balance, however, there are probably more benefits associated with deeper ponds (eg, ease of fish harvest, water conservation, more stable temperatures, reduced effect of sedimentation on interval between renovations).
Increase pond depth
Obviously, deeper ponds contain more water than more shallow ponds. Therefore, at a given feeding rate, deeper ponds should have lower ammonia concentrations because there is more water to dilute the ammonia excreted by fish. In reality, deeper ponds do not usually have enough water to significantly dilute ammonia when compared to the large amounts of ammonia in constant flux between various biotic and abiotic compartments in ponds. Furthermore, deeper ponds are more likely to stratify and the lower layer of pond water (the hypolimnion) can become enriched with ammonia and depleted of dissolved oxygen. When this layer of water mixes with surface water in a “turnover,” severe water quality problems may result.
Flush the pond with well water
Ammonia can be flushed from ponds, although pumping the huge volume of water required to do so in large commercial ponds is costly, time-consuming and unnecessarily wasteful. It is also deceptively ineffective as an ammonia management tool. For example, assume the ammonia concentration in a full, 10-acre pond is 1 mg/L. The ammonia concentration after pumping 500 gpm continuously for 3 days (equivalent to about 8 inches of water) will be 0.90 mg/L, a drop of only 0.10 mg/L.
Instead of simply running water through a pond as in the example above, now assume that about 8 inches of water is discharged from the pond before refilling with well water. In this case, the decline in ammonia concentration will be slightly greater (to 0.83 mg/L), but even this decrease is not enough in an emergency situation, particularly when the extra time needed to drain the water before refilling is considered. The difference in the two flushing scenarios is related to the blending of pond water with pumped water before discharge in the first case.
Just as paddle wheel aeration creates a zone of sufficient dissolved oxygen concentration, pumping groundwater creates a zone of relatively low ammonia concentration adjacent to the water inflow. The effectiveness of this practice is questionable because it does not address the root cause of the problem and wastes water. Flushing ponds is not only ineffective, but highly undesirable because of concerns about releasing pond effluents into the environment.
Add bacterial amendments
Common aquatic bacteria are an essential part of the constant cycling of ammonia in a pond ecosystem. Some people believe that ammonia accumulates in ponds because the wrong kind or insufficient numbers of bacteria are present. If this were true, adding concentrated formulations of bacteria would address the problem. However, research with many brands of bacterial amendments has consistently given the same result: Water quality is unaffected by the addition of these supplements.
Standard pond management creates very favourable conditions for bacterial growth. Bacterial growth and activity is limited more by the availability of oxygen and by temperature than by the number of bacterial cells. Also, the most abundant type of bacteria in many amendments (and in pond water and sediment) is responsible for the decomposition of organic matter. Therefore, if bacterial amendments accelerate the decomposition of organic matter, ammonia concentration would actually increase, not decrease.
Another kind of bacteria in amendments oxidises ammonia to nitrate. Adding them will not reduce the ammonia concentration rapidly because the bacteria must grow for several weeks before there is a large enough population to affect ammonia level.
Add a source of organic carbon
If the dissolved oxygen concentration is adequate, adding a source of organic carbon, such as chopped hay, to intensive fish ponds can reduce ammonia concentration. Many bacteria in fish ponds are “starved” for organic carbon, despite the addition of large amounts of feed. Organic matter in fish ponds (dead algae cells, fish fecal solids, uneaten feed) does not contain the optimum ratio of nutrients for bacterial growth. There is more than enough nitrogen for bacterial growth so the excess is released to the pond water.
Adding organic matter with a high concentration of carbon relative to nitrogen promotes the “fixation” or “immobilisation” of the ammonia dissolved in water. Incorporating ammonia into bacterial cells packages the nitrogen into a particulate form that is not toxic to fish. The down side of this approach is that it is hard to apply large amounts of organic matter to large ponds and the effect on ammonia concentration is not rapid. Furthermore, aeration will have to be increased to address the demand for oxygen by large quantities of decomposing organic matter.
Add ion exchange materials
Certain naturally occurring materials, called zeolites, can adsorb ammonia from water. These are practical to use in aquaria or other small-scale, intensive fish-holding systems, but impractical for large volume fish ponds.
Some shrimp farmers in Southeast Asia have tried making monthly applications of zeolite at 200 to 400 pounds per acre. However, research has demonstrated that this practice is ineffective at reducing ammonia concentration in ponds and it has now been abandoned.
Add acid
In theory, adding acid (such as hydrochloric acid) to water will reduce pH. This can shift the ammonia equilibrium to favour the non-toxic form. However, a large amount of acid is necessary to reduce the pH in well-buffered ponds and it would have to be mixed rapidly throughout the pond to prevent “hot spots” that could kill fish. Furthermore, adding acid would destroy much of the buffering capacity (alkalinity) of the pond before any change in pH could occur. Once the ammonia concentration is lowered, treated ponds might require liming to restore the buffering capacity. Working with strong mineral acids is a safety hazard for farm workers and for fish.
How often should ammonia be measured?
From the foregoing discussion, you might assume that measuring ammonia in ponds is unnecessary. After all, research has indicated that brief daily exposure to ammonia concentrations far higher than those measured in commercial ponds does not affect fish growth. And, on the rare occasions when ammonia does become a problem, there is nothing you can do about it. However, there are some special circumstances when it is worthwhile to monitor ammonia levels.
In the southern United States, ammonia concentrations in most ponds usually start increasing in September and peak about mid-October, about 5 to 6 weeks after the last stretch of high feeding rates. Then, about 2 to 4 weeks later, nitrite concentrations peak. This is a general pattern. It does not apply to all ponds, and ammonia or nitrite problems can occur with variable intensity at any time, especially between September and March.
Thus, the magnitude of the ammonia elevation in the early fall can indicate the severity of the nitrite spike that will follow. Salt can protect fish against nitrite toxicosis (see SRAC Publication No. 462). If enough salt is added to ponds to achieve chloride levels of 100 to 150 mg/L, there is no reason to measure ammonia even as a predictor of high nitrite concentrations.
Ammonia should be measured every other day after the crash of an algae bloom and weekly in the cooler months of the year to identify ponds that may have a potential problem with nitrite. Other than those times, it is probably not necessary to measure ammonia in fish ponds.
To summarise, fish producers should not be alarmed if ammonia concentration becomes elevated, although a high ammonia level often indicates that nitrite concentrations may soon rise. In this case, farmers should focus on protecting fish from nitrite poisoning by adding salt, rather than on trying to manage the ammonia problem. Extra vigilance after an algae crash is also probably warranted. Usually, the concentration of ammonia will fall again once the bloom becomes re-established.
Because there is little that can be done to correct problems with ammonia once they occur, the key to ammonia management is to use fish culture practices that minimise the likelihood of such problems. This means stocking fish at a reasonable density, harvesting as often as practical to keep the standing crop from being too large, and using good feeding practices that maximise the proportion of the feed consumed by fish.
Click here to read about managing ammonia spikes when farming shrimp.




