Salmon lice epizootics, i.e. infestations exceeding expected natural occurrences, on wild fish are one of the major causes for concern regarding the environmental sustainability of the open net pen salmon farming industry (Gargan et al. 2006, Krkosek et al. 2007, Middlemas et al. 2013).
Salmon lice are natural parasites on wild salmonids throughout the Northern Hemisphere (Boxshall 1974, Boxaspen 2006). However, intensive fish farming of Atlantic salmon and rainbow trout has led to an increase in potential hosts.
This again has led to a shift in parasite host dynamics, increasing the likelihood for the parasite to complete its life cycle and simultaneously augmenting the chance that wild hosts can become infested by lethal numbers of parasites (Costello 2006). Sea trout Salmo trutta L. are usually found close to the coast, feeding in shallow nearshore habitats during their sea sojourn.
This is also an area where infectious stages of salmon lice aggregate (Penston et al. 2008a,b). The few studies that have registered salmon lice on sea trout in areas with no salmon farming generally report occurrences of high prevalence, but generally low intensity (Tingley et al. 1997, Schram et al. 1998).
Epizootics have been registered in areas with fish farming (Heuch et al. 2009, Bjørn et al. 2011, Serra-Llinares et al. 2014). However, typical of these epizootics is that they occur during spring to autumn and often during periods of relatively high temperatures.
One undocumented concern has been whether salmon farming has led to an increase in infestation levels in periods of the year when sea trout do not naturally encounter large numbers of salmon lice.
In areas without fish farms, salmon lice populations go through a bottleneck during winter months due to low temperature and relatively few hosts (Heuch et al. 2002).
In cases where fish farms are present, numbers of hosts can, in theory, curtail this bottleneck and create epizootics on wild fish even at lower temperatures. Here we report on the first observations of winter epizootics of salmon lice Lepeophtheirus salmonis on sea trout in Sognefjorden located in western Norway.
Results
In 2013, relatively low numbers of salmon lice were counted during field observations. In general, only adult stages were recorded from 28 January until 27 March (N = 58, intensity = 5.78, max = 26, prevalence = 81%, Kvamsøy).
In May, an increase in the attached stages was reported (4 April to 4 June, N = 294, intensity = 19.5, max = 110, prevalence = 13.6%, Ligtvor). We therefore decided to take a randomized sample from the trap net (i.e. all fish caught were sampled except for fish > 50 cm; the latter were released with minimal handling to avoid killing large and old individuals).
Sampled fish were caught between 13 May and 8 June. The results from analysis in the laboratory confirmed that there were low numbers of salmon lice on the sea trout during this period (N = 55, intensity = 6.16, max = 23, prevalence = 34.5%).
In 2014, relatively high abundances of attached stages were reported during field observations in early March at both Kvamsøy (1 March to 20 March, N = 32, intensity = 23, max = 220, prevalence = 72%) and Kyrkjebø (12 March to 24 March, N = 27, intensity = 16, max = 100, prevalence = 67%).
We therefore took a randomized sample from each of the 2 trap nets (i.e. all fish caught were sampled except for fish >50 cm). Sampled fish were caught between 9 March and 24 March. In Kvamsøy, the prevalence was 100% (N = 12), with a mean intensity of 254.3 (±296.7 SD; Table 1).
Fifty-four percent had more than 0.1 lice g–1 and 73% had more than 10 lice fish–1. Some of the individuals had very high infestation levels, with a maximum of 759 lice on a 351 g fish. The stages were dominated by chalimus 1 (53.3%) and chalimus 2 (37.8%). There were relatively few preadults (4.0%) and adults (1.2%).
Copepodites were also low in number (3.6%), but we cannot be certain that some of these were not wrongly characterized, as copepods can be overlooked due to the high number of chaimus stages on some of the fins. In Kyrkjebø, the prevalence was 73% (N = 14), with a mean intensity of 37.5 (±56.7 SD, max = 188; Table 1).
Fourteen percent had more than 0.1 lice g–1 and 36% had more than 10 lice fish–1. Similarly to Kvamsøy, the stages were also dominated by chalimus 1 (11.7%) and chalimus 2 (52.9%). However, there was also a high percentage of preadults (24.4%), with relatively few copepodites (2.7%) and adults (2.7%).
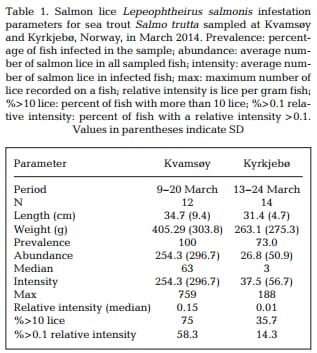
There were more attached stages (ANCOVA, F1,23 = 8.51, p < 0.05) and mobile stages (ANCOVA, F1,23 = 11.01, p < 0.05) on the fish sampled in Kvamsøy compared to Kyrkjebø. For mobile stages, the number of lice increased with the length of the fish ANCOVA, F1,23 = 6.78, p < 0.05).However, this was not the case for attached stages (ANCOVA, F1,22 = 1.05, p > 0.05; Fig. 2)
Discussion
Infestations on sea trout similar to those observed in March in 2014 have been recorded during the spring and summer months in areas with high farming activity (Heuch et al. 2009, Bjørn et al. 2011, Serra-Llinares et al. 2014), and anecdotal information suggests that epizootics can also occur in areas without fish farms during periods of high water temperature and low freshwater run-off.
The surface temperature (within 1 m) at the fixed hydrographic station in Sognesjøen (outer region of Sognefjorden) during January and February was 6.4 and 3.5°C in 2013 and 6.1 and 5.7°C in 2014 (6.3 and 5.5°C on average for the period 2000− 2012, www.imr.no), respectively.
Consequently, the temperature was well below temperatures that routinely occur during the summer and early autumn months in Sognefjorden (July average = 15.4°C).
According to the model by Stien et al. (2005), it will take more than 28 d for individuals to reach the chalimus 1 stage at 5.7°C. This suggests that the production of the main proportion of the lice observed on the fish occurred in January or February. Natural salmon lice intensity during winter months on sea trout is usually not above 3 lice per fish, with a prevalence seldom exceeding 20% (Schram et al. 1998, Rikardsen 2004).
Consequently, the prevalence and abundance observed here are above normal and can be defined as a winter epizootic.
One likely source of salmon lice on sea trout in Sognefjorden is lice produced on salmon farms. Correlations between lice counts and biomass in farms and counts of lice on sea trout have been corroborated in various studies (Middlemas et al. 2013, SerraLlinares et al. 2014).
In general, the correlation is dependent on the distance from the fish farms, decreasing from 0 to 30 km from the closest farm, but with large variation around this cut-off point (Middlemas et al. 2013).
The source of this variation is most likely that the interaction between infestations on wild and farmed fish is dependent on a number of factors, such as behaviour, local topography and currents (Brooks 2005).
In our study, the infestation levels were higher on fish from the location furthest from fish farms within the National Salmon Fjord (Kvamsøy). This location is also further into the fjord, and the high infestation levels on these fish may be a result of individuals moving towards lower salinity water to avoid further infestations or behaviorally regulate osmoregulation (Birkeland & Jacobsen 1997).
The effects on the populations of sea trout from salmon lice epizootics during winter months are uncertain. Sea trout can descend rivers throughout the year (Jonsson & Jonsson 2009). While some individuals only stay in the ocean for days to months during spring and autumn, some sea trout also reside in the ocean throughout the winter, and in a few cases up to several years before ascending (Jonsson & Jonsson 2009, Jensen & Rikardsen 2012).
This sea sojourn strategy may be an important component of the life history strategy of sea trout in order to ensure population stability. In some rivers and creeks, sea trout are only found in the river during the spawning season, residing most of the time in the estuary or in marine waters (Knutsen et al. 2004, Olsen et al.2006).
This is most likely because the freshwater habitat can be variable and in some cases detrimental. For example, habitats may temporally dry out during recurrent drought events, and in northern latitudes, smaller watersheds can freeze and become uninhabitable during the winter months (Limburg et al. 2001, Jensen & Rikardsen 2012).
Consequently, increased salmon lice infestation during the winter months may be detrimental for some populations and life history strategies in sea trout. The general trend in reports from sea lice counts from fish farms in Norway has been a decrease in the abundance of lice during spring months in the last decade, following an increased focus on lowering the production of salmon lice during the migration of salmon smolt (Jansen et al. 2012).
However, simultaneously, an increase in the number of lice during the< winter months has been reported in the last several years (Jansen et al. 2012). The present study is the first report of a winter epizootic of salmon lice on sea trout in Norway. No surveillance exists to document how frequent such winter epizootics are along the Norwegian coast. We highly recommend a more thorough investigation to document the severity of such outbreaks on wild fish.
October 2014



