Using chemicals to treat parasitic diseases in farmed fish can be risky, for both human health and the environment, while concerns remain over antibiotics and antimicrobial resistance. In light of this, natural treatments – or biocontrols – are increasingly drawing attention.
© David B Vaughan
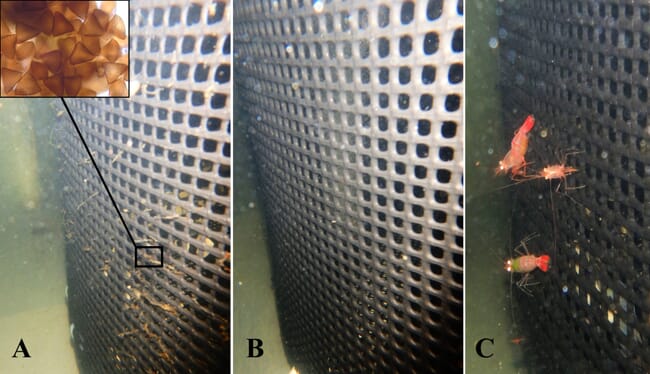
One promising candidate is the peppermint cleaner shrimp (Lysmata vittata), which, according to scientists in Australia, could play a vital role in reducing parasites in farmed fish. Using grouper as their test-subject species, the scientists found that the shrimp reduced infection by the parasite Neobenedenia girellae by 87 percent. Their study is the first to examine the potential of shrimp as a biocontrol agent in aquaculture using RAS with grouper. It follows an initial investigation of the species Lysmata amboinensis against Neobenedemia girellae in 2015.
“We investigated cleaner shrimp because of their potential to remove fish ectoparasites and we wanted to learn more about their ecology and use as a possible biocontrol,” says Dr David Vaughan, a biologist at James Cook University (JCU) in Townsville, Australia, who worked on the study with Dr Kate Hutson at JCU’s Centre for Sustainable Tropical Fisheries & Aquaculture and Dr Alexandra S Grutter at the University of Queensland. “We chose grouper because they’re an important high-value fish farmed throughout the Asia-Pacific region, the area of greatest aquaculture production globally, and are greatly affected by parasites.”
Using natural predators to control parasites isn’t a new idea in aquaculture. Wrasse and lumpfish are used in salmon farming to control sea lice but there is no equivalent biological control for tropical or subtropical aquaculture. Believing that cleaner shrimp could offer an answer, Dr Vaughan and his colleagues used four species to determine their individual performance at removing three different parasites from host fish. Each shrimp fed on each parasite twice – once on adult parasites that had infected the skin of grouper and once on the parasites’ eggs, cysts or cocoons.
The peppermint cleaner shrimp was then tested under recirculating-aquaculture conditions using grouper infected with Neobenedenia girellae. The shrimp removed and consumed the eggs of the parasite from the sides of the fish cages.
“The peppermint cleaner shrimp is a gregarious, widely distributed species that eats economically important fish parasites like monogeneans, leeches and protozoans,” says Vaughan. “The fact that it also eats the environmental stages of these parasites – ie eggs, cysts and cocoons – is important, given that these are generally impervious to most chemical treatments used to treat the on-fish stages and are therefore the main cause of reinfections. I was very surprised by how efficient the shrimp were.”

© Richard Knuckey
Richard Knuckey is managing director at The Company One, home to Australia’s only grouper hatchery. He says that compared to areas like South-East Asia, where the flow of live grouper from fingerlings to market size is extensive, Australia has more legislations and processes to prevent the spread of disease, such as fish-health monitoring, water sterilisation and free government diagnostic services. But very few therapeutic agents are approved for use with fish.
“In this sense, alternative, chemical-free disease-control measures are worth exploring,” he says. Using cleaner shrimp instead of wrasse or lumpfish may have less biosecurity risk, as they’re unlikely to be hosts for some key grouper pathogens. The difficulty will be making the method practical for on-farm adoption. Acquiring enough and maintaining populations of the cleaner shrimp would be the follow-on research after assessment of their effectiveness.”
Vaughan’s work is in the early stages; all studies were conducted under laboratory conditions and have not been tested in the field. On-farm trials are now required, as well as additional studies with different shrimp from temperate locations. More parasite species will also need to be included to see if additional shrimp species can be used in different aquaculture regions that also suffer from parasite problems. But Vaughan sees the potential for cleaner shrimp.
“They aren’t susceptible to the ectoparasites of their client fishes,” he said. “This is an advantage, especially when considering the diversity of fish parasites in tropical aquaculture that have a low host-specificity. Different shrimp species should be targeted for testing against some of the major parasitic impediments in fish, bivalve and mollusc farming, and be investigated further as a means of control against biofouling.”

© Mike Rimmer
Mike Rimmer is a senior research fellow in aquaculture at the School of Science and Engineering at the University of the Sunshine Coast (USC), Australia and co-author of a recent review of grouper aquaculture. He explains that the main technique to control parasites in grouper culture is freshwater bathing, when fish are netted from sea cages and placed in freshwater for periods of up to two minutes. While this removes ectoparasites, there appears to be no information on areas such as efficacy, optimal duration of the bath or frequency of use.
“In this sense, Dr Vaughan’s study results are very interesting, and cleaner shrimp may have application with grouper raised in RAS systems where escapement isn’t an issue and there is less chance of reinfection,” he says. “In sea-cage culture, many of the shrimp may simply swim away to find a preferred habitat. Reinfection is also an issue in sea cages due to the large populations of incidental resident fish outside the cages.”
“There is also the issue of cost of the cleaner shrimp,” he continues. “Currently they’re quite expensive. The market for ornamental fish is quite happy to pay high prices but grouper farmers in South-East Asia are always seeking to reduce costs and would want cheaper options.”

© Richard Knuckey
“This technology could be applied to maintaining high-value broodstock in hatcheries such as ours,” says Knuckey. “These fish are kept in clean conditions under a controlled environment so it’s likely that the shrimp would thrive and be beneficial in controlling skin parasites. In RAS operations, the density of fish and water-exchange rates are also high. It would be challenging to establish sustainable cleaner-shrimp stations in these systems and their tolerance for these environmental parameters has not been assessed. For pond and sea-cage grouper grow-out, the shrimp could thrive and be effective, but their adoption would rely on access to a plentiful and cost-effective supply. The study has demonstrated the potential of cleaner shrimp to control a range of parasites. While more work is required to address the practicalities, alternative, low environment impact methods of disease management must be found.”
“The cost and/or supply of peppermint cleaner shrimp are valid concerns, and the more that are produced, the cheaper they will become,” says Vaughan. “They’re a domesticated species already and currently produced in Tasmania for the ornamental industry. The technology to produce them is available, and they are also produced at James Cook University.”
The peppermint cleaner shrimp could be an ideal candidate but the risks of particular diseases are often specific to location, species, farming methods, biosecurity and other mitigation strategies. Vaughan recommends that grouper farmers educate their staff in effective biosecurity to further reduce the impact of disease, and acknowledges that more must be done to determine the best way to use cleaner shrimp in a commercial setting.
“If their use could be shown to provide a direct competitive advantage for farmers, they would consider it. Farm trials are necessary to demonstrate this potential,” he concludes.



