Introduction
Aquaculture is one of world's growing
animal production segments. In Brazil,
however, commercial fish production in
net pens is only just starting, despite its
great potential represented by 6 million
hectares of water contained in weirs
and reservoirs constructed mainly for
generating hydroelectric energy. In the
future, Brazil is likely to become one of the
world's largest aquaculture producers.
Among fish species showing potential for
cage farming is Nile tilapia (Oreochromis
niloticus). Over the past decade, it has
become the species with the largest
production volume in Brazil, representing
nearly 40% of the country's aquaculture.
Tilapia cultivation has been developed
primarily in Brazil's South, Southeast and
Northeast regions, the latter representing
the highest production volume. In 2004,
41% of total domestic tilapia production
occurred in this region. The Northeast
region, in fact, has led the country's
tilapia production since 2003, with a clear,
growing trend due to its climate and
technological development, enabling it
to serve the growing demand for tilapia
both regionally and nationwide.
In recent decades, improved technology in
fish production units has become a major competitive advantage. Efforts to survive
and withstand an increasingly globalized
market have become an evident need. In
this context, and despite efforts to improve
fish quality through the implementation of
health programs and novel technologies,
tilapia production success depends on
innovative tools.
A future challenge can now be foreseen:
Mankind demands more and more
products that are not only nutritious
but are wholesome and pathogen-free,
that promote health and that are
environmentally friendly and socially fair
in perfect harmony with the globalized
world we live in today.
Intensive fish production, however, brings
about stress, resulting in the emergence of
diseases and, therefore, mortality.5 Among
the major maladies affecting tilapia,
Streptococcus agalactiae infection, resulting
in streptococcosis, plays a very important
role worldwide.6 Epidemiological studies
sponsored by MSD Animal Health around
the world have shown the presence of two
different S. agalactiae groups or biotypes
(i.e., I and II). Tilapia isolates from different
regions in the world show that 26% of
streptococci were identified as S. agalactiae
Biotype I, while 56% were S. agalactiae
Biotype II. S. agalactiae Biotype II is the
world's most prevalent biotype, found
mostly in China, Indonesia, Vietnam, the
Philippines and Latin America. In Brazil,
serological studies show 100% positive
serology to S. agalactiae Biotype II.
The largest economic impact due to
S. agalactiae in freshwater-farmed fish
species occurs in Nile tilapia. The
geographic distribution of S. agalactiae
includes regions with temperate, tropical
weather, where warmwater fish are
cultivated. So far, outbreaks have been
reported in several countries including:
the US, Japan, Kuwait, Israel, Thailand
and Brazil.
This pathogen is responsible for high
economic losses; mortality on a farm can
reach 90%, typically at the pre-market
age when substantial feed volumes
have already been consumed. We must
remember that feed is the largest
component of production costs and that
much has been invested in the fish by
this time.
Infection occurs when infected fish
dead or alive, moribund or apparently
healthy release the bacterium into
the water, allowing it to colonize the skin
of other fish. Invasive infections can also
occur, resulting in high mortality. In
addition, the bacterium can survive for
long periods of time in water, mud or
pond/greenhouse substrates, and
even on pieces of equipment used in
routine operations.
If tilapia culture is to continue and proliferate,
the industry will need strategies to
minimize the effects of disease. The advent
of S. agalactiae vaccines has brought
about a new, promising tool, since some S. agalactiae field strains have already developed
resistance to antimicrobials.9 Evaluation
of the vaccine AquaVac Strep Sa
under experimental conditions has demonstrated
significantly decreased
mortality in vaccinated fish, illustrating
the efficacy of the vaccine for the
prevention and control of streptococcosis
in Nile tilapia.
Materials and Methods
In the study, 180 Nile tilapia (Oreochromis
niloticus) juveniles from the same spawn
and weighing ~35 g were used. Prior to
the start of the study, the fish were
maintained in quarantine and subjected
to a prophylactic antimicrobial bath;
they were then conditioned in 250-liter
cages under constant dechlorinated
water exchange.
Upon the completion of quarantine,
fish were moved to the Aquatic
Organism Experimental Infection Unit,
Fish Immunopathology Laboratory (Unidade de Infeco Experimental de Organismos
Aquticos do Laboratrio
de Imunopatologia de Peixes, LIPPE) and
conditioned in 12, 80-liter aquariums
(n = 15). Aquariums received UV-sterilized,
dechlorinated, running water from an
artesian well. Fish acclimation lasted 7
days, which was time enough for plasma
cortisol concentrations and osmolality
to return to baseline levels.
Water temperature was measured daily
(27 C/81 F 1.5). Hydrogen ion potential
(7.1 0.3) and dissolved oxygen (5.5 mg/L
1 mg/L) were measured weekly. All
values remained within welfare-recommended
levels.
Throughout the experiment, ad libitum
feeding was provided twice daily (09:00h
and 17:00h) at the rate of 5% of biomass.
Experimental Design
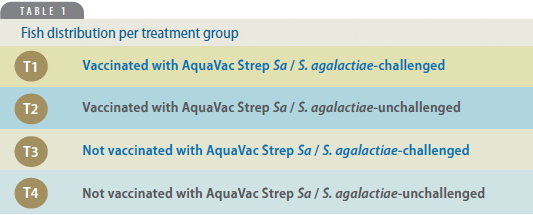
Fish in the 12 aquariums represented three repetitions for each treatment group (Table 1).
Vaccination
Vaccination against S. agalactiae was performed at the end of the acclimatization period at the laboratory. Fish weighing ~35 g (medium bodyweight) were submitted to the vaccination process as recommended by MSD Animal Health. The fish were anesthetized by immersion in 1% eugenol (Biodinamica) containing 50 mg/L water. A single 0.05 mL dose of AquaVac Strep Sa was injected intraperitoneally in the anterior-medial aspect, using a sterilized insulin syringe/needle.
Challenge
Challenge was performed 25 days
after vaccination. For inoculum
preparation, live streptococcus isolates
from naturally infected tilapia were
used. Isolates were previously classified
as Lancefields B group, using the
Slidex Strepto Kit latex agglutination
test (BioMerieux, France), then
characterized as S. agalactiae based
on phenotype characteristics as
determined by the ApI 20 Strep
Microtest System (BioMerieux, France.)11
The selected S. agalactiae strain was
seeded in brain/heart infusion broth,
incubated for 24 hours at 29 C (84.2 F) under aerobic conditions. The challenge
dose (106 colony-forming units/mL) was
calculated on the basis of a lethal dose
killing 50% of the fish population (LD50).
Fish were observed for 15 consecutive days after challenge for clinical signs and
death; the findings were recorded daily
and subjected to statistical analysis
(Tukey's test), with a 5% level
of significance.
Results
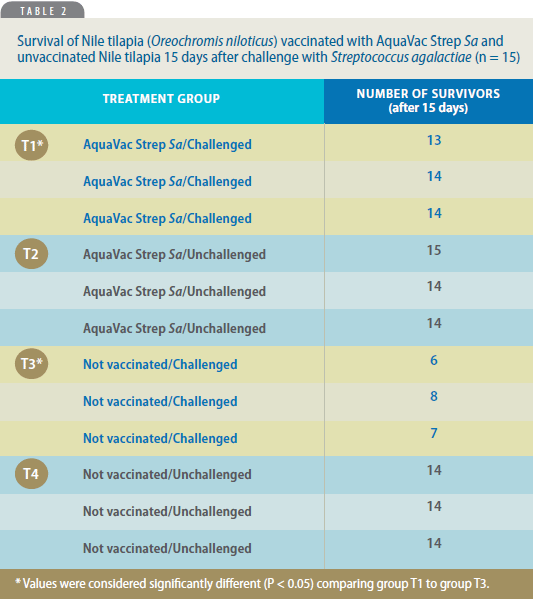
Table 2 shows survival among fish in
various treatment groups. No significant
mortality occurred when comparing
groups T1 (vaccinated/challenged) and
T2 (vaccinated/unchallenged) with group
T4 (not vaccinated/unchallenged),
demonstrating the vaccines safety. None
of the fish that died in those groups
showed clinical signs compatible with
streptococcosis, but deaths occurred
within 48 hours after challenge, suggesting
that the cause of death was handlingassociated
stress.
Animals in group T3 (not vaccinated/
challenged) that died showed clinical signs
compatible with streptococcosis, mostly
after 7 days post-challenge. Clinical signs
included lethargy, reduced appetite,
body-color darkening, uni/bilateral
exophthalmia, abdominal distention and
erratic/circular swimming. Necropsy
findings among these fish included ascites,
enlarged livers and diffused hemorrhages
in the central nervous system. Upon
microbiological analysis, S. agalactiae was
re-isolated, particularly from the brain,
which means generalized infection.
The appearance of clinical signs after 7
days post-challenge represents the natural
evolution of the infection. Neurological
signs suggest meningoencephalitis, and it represents a clinical sign consistent with
streptococcosis. The mean number of
deaths among repetitions supports the
LD50 calculated for this particular study.
Variations in the numbers of dead fish
among repetitions can be the result of
innate, individual resistance variability.
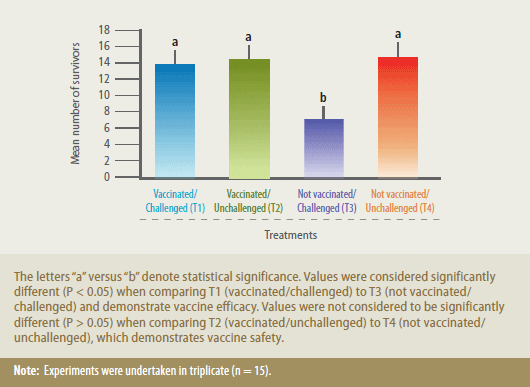
Vaccination with AquaVac Strep Sa
protected animals against experimental
challenge with S. agalactiae, since mortality in group T1 (vaccinated/challenged) was
significantly lower (P < 0.05) than mortality
in the T3 (not vaccinated/challenged) fish
(Figure 1). In this context, the relative
protection percent (RPP) was 84%. RPP was
determined using the following equation:
RPP = (1- (vaccinated fish deaths/control
fish deaths)) x 100.
Mean Number of Survivors in Nile Tilapia (Oreochromis Niloticus) Vaccinated with AquaVac Strep Sa then Challenged with Streptococcus Agalactiae
Conclusion
Results from this study demonstrate that AquaVac Strep Sa induced effective protection in Nile tilapia experimentally challenged with S. agalactiae. We, therefore, conclude that AquaVac Strep Sa (MSD Animal Health) is a safe and highly efficacious vaccine against the disease caused by S. agalactiae Biotype II. A single dose of AquaVac Strep Sa, administered as directed, can be an important tool in the prevention and control of streptococcosis in Brazil, since serological results so far show only the presence of Biotype II in this country.
August 2012
This article contains information on veterinary pharmaceutical and biological animal health products based on international registration dossiers. It may refer to products that are either not available in your country or are marketed under a different trade name. In addition, the safety and efficacy data and the withholding periods for a specific product may be different depending on local regulations. Consult the the regulatory and technical information on available veterinary drugs in your country.



