The barramundi (also known as the Asian seabass Lates calcarifer) is native to the Indo-Pacific region and is well suited to aquaculture. Although most of the current production is consumed domestically, due to its tasty, firm white flesh, barramundi is rising in popularity as a seafood item in the US and Europe since the late 1990's. Recently, several large international aquaculture operations have been set up in South East Asia to produce barramundi to take advantage of the suitable growing conditions and lower labour costs. The species is however not without its problems. Diseases are a major concern for the future sustainability of this and indeed any aquaculture industry.
Building upon extensive experience in salmonids, Intervet/Schering-Plough Animal Health established a research centre in Singapore in 2000 dedicated to the development of novel vaccines and other products for commercially important farmed warm water species.
Graph 1. Strain collection data base*; barramundi bacterial isolates since 2000. (NB. not true epidemiology as some countries were visited more frequently than others)
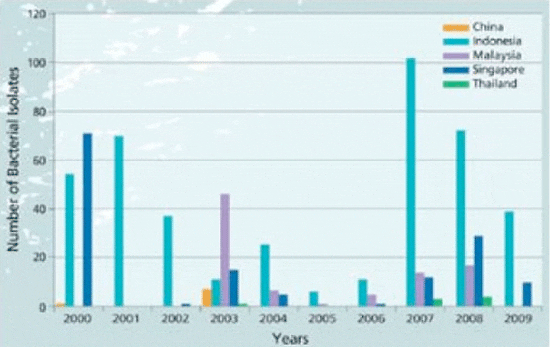
Chart 1. An overview of the major diseases of farmed barramundi in South East Asia.
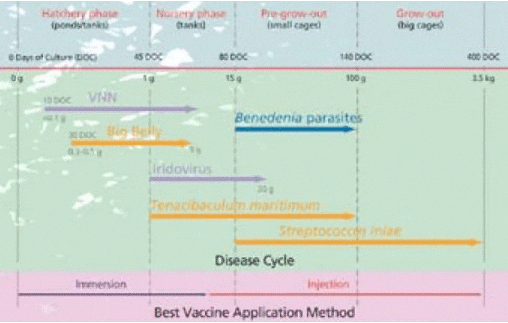
The company identified barramundi as a major farmed species for the region and therefore initiated extensive epidemiology surveys. Over the last 8 years, this routine surveying has resulted in a stored bank of barramundi-specific bacterial isolates from Indonesia, China, Malaysia, Singapore and Thailand (Graph 1) and has allowed the team in Singapore to identify the most important diseases in this species (summarised in Chart 1).
This article will highlight each of the major diseases in barramundi and their specific characteristics.
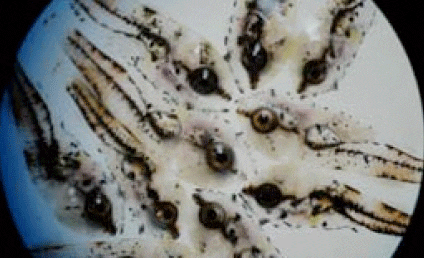
Viral Nervous Necrosis (VNN) is acute in larvae that are ten days old and often results in 100% mortality. The transparent larvae (due to the contraction of chromatophores) will display corkscrew or whirling swimming patterns and have hyper-inflated swim bladders.
Picture 1: VNN infected barramundi larvae, transparent with contraction of chromatophores
Picture 2: H&E stain (*200) Characteristic Viral Nervous Necrosis vacuoles present in retina of an infected fish
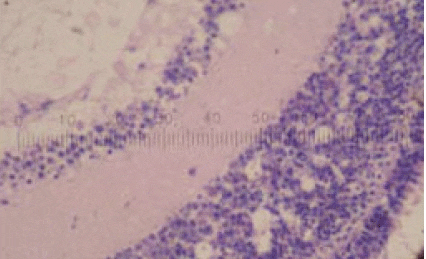
The clinical signs are very characteristic of the disease. Histopathological sections clearly illustrate pathogonomic cell vacuolisation and necrosis of the central nervous system with lesions also occurring in the retina and spinal cord. A combination of histopathology and nested Polymerase Chain Reaction (PCR) will confirm the outbreak.
The disease can be passed either horizontally through the broodstock with evidence of the virus detected in brood-fish gonads, fertilised eggs, and larvae or vertically through the water with VNN outbreaks in fish farms occurring after introduction of infected juveniles. There is also possible transmission between wild and farmed fish.
There is no specific treatment; however, ozone treatment of all incoming eggs and larvae has been shown to reduce transmission potential. Development of VNN free broodstock with broodstock screening and isolation will be an important strategy in managing this disease.
Big Belly Disease is an intracellular, bi-polar large coco bacillus bacterium characterised by the company in 2004. It is present in several South East Asian countries including Indonesia, Singapore and Malaysia. To date, the disease has only been isolated from barramundi.
Big Belly is typically severe in 25-day-old fry in the nursery causing severe clumping of internal organs, abdominal distension and muscular atrophy. Often, infected individuals will take on a darker colouration, become lethargic, separate from the ‘school' and lose equilibrium with either surface swimming or resting on the bottom of tanks. Considered to be a slow systemic infection, onset of mortality is gradual but consistent and without treatment has the potential to kill 90-95% of the population.
Picture 3: Typical ‘knife edge' tail and abdominal distension characteristic of Big Belly in juvenile barramundi

Picture 4: Impression print of large bi-polar coco-bacillus bacterium.
Diagnosis in the field is based on the occurrence of clinical signs with a typical ‘knife edge' to the tail (Picture 3) and a glutinous mass of intestines. Also, impression prints of visceral fat and other intestinal material will reveal the characteristic form of the bacterium (Picture 4).
Isolation of the bacterium is difficult due to its intracellular nature and the need for a specific agar medium to inhibit most of the environmental contamination. The R&D group in Singapore has developed Big Belly specific primers for PCR amplification.
In the field Aquaflor®, a premix formulation containing 50% active florfenicol, has shown promising results in reducing mortality due to the disease.
Iridovirus is one of the most severe diseases of tropical marine species such as barramundi and grouper (Epinephelus spp) but also affects many other fish species including some in freshwater. In barramundi the disease mainly occurs in fish of 10 to 50g and causes acute peaks of mortality of up to 80 - 90% (Picture 5). The fish will turn black and lose appetite. On closer clinical examination the gills will appear very pale and may bleed when handled and blood may also leak into the iris giving a ‘red eye' appearance ( Picture 6). Internally, a pale spleen is characteristic of this disease.
Picture 5: Acute mortality peaks typical in barramundi leave few survivors

Picture 6: ‘Red eye', an often-typical clinical sign of Iridovirus in barramundi juveniles because of massive internal haemorrhaging

Picture 7: Electron microscopy of grouper Iridovirus illustrating typical icosahedral viral particles
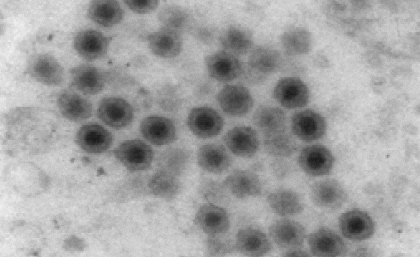
Mortality peaks and indicative clinical symptoms are generally good indicators of this disease in the field. Electron microscopy of spleen and kidney tissue reveals icosahedral viral particles typical of iridoviruses (Picture 7) and may be confirmed by PCR analysis using primers against the major capsid protein. Conventional treatment strategies have so far not proved effective.
Parasites It is almost impossible to avoid parasites because usually they are present as part of the aquatic ecosystem. Health monitoring and early diagnosis is key for control of parasitic diseases. Protozoa (particularly Cryptocaryon irritans and Trichodina spp.) and capsalid monogeans on the body surface (particularly Neobenedinia spp.) most commonly affect newly stocked barramundi in open ocean cages. Current observations and reports from South East Asia indicate that capsalid monogean infections are the most serious and pathogenic amongst all of the parasitic diseases. If left untreated barramundi quickly develop skin and tail rot and mortality rates of 30-40% are common.
Neobenedinia most commonly affects younger fish but if a population has been compromised in anyway (even from the nursery stage) then they will always be susceptible. Typically, a proportion of the fish will be ‘off feed' and lethargic. The parasite also irritates the eyes causing opacity and exophthalmia (‘pop eye') and gradually the caudal and pectoral fins will become frayed (white appearance in the water) and hemorrhagic when handled.
Picture 8: Dorsal fin of a barramundi illustrating opaque Neobenedinia parasites after immersion in freshwater

Picture 9: Indonesian farmers performing a bath treatment in response to a Neobenedinia outbreak in barramundi

Neobenedinia is particularly easy to spot at the farm because after the whole fish is immersed in freshwater for some minutes, the parasites will turn opaque (Picture 8). A specific parasite prevention program with routine freshwater immersion, skin scrapes and gill clips should be considered an integral part of the health management protocols.
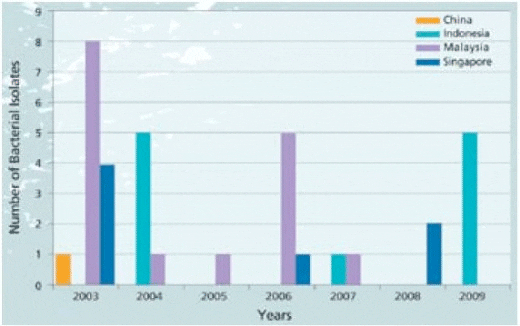
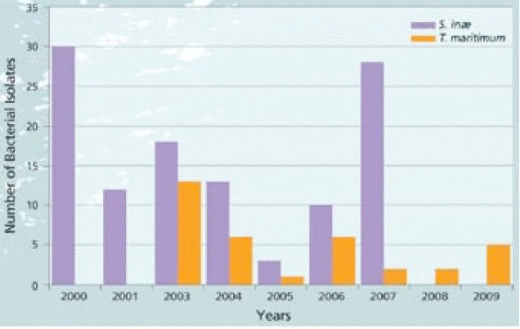
Bacteria Tenacibaculum maritimum (T. mar) Tenacibaculum maritimum, also described as T. mar, is a rod shaped gram negative, filamentous bacterium. In Singapore, the team has been working with this pathogen since 2003 (Graph 2). There are 95 isolates from China, Indonesia, Malaysia, Singapore, Korea, Japan and Greece stored in the laboratory. The majority of isolates come from tropical saline species including barramundi, snapper (Lutjanus spp), grouper, cobia (Rachycentron canadum) and pompano (Trachinotus blochii).
T. mar can be especially severe when combined with skin parasites and outbreaks of the disease often occur after a stressful event. Typically, a T. mar outbreak will start as small lesions on gills and the ventral side of the fish, resulting in chronic scale loss and spreading to other cartilaginous body parts including the face and jaw.
Associated mortality in barramundi may be gradual or acute depending on the nature of the outbreak but often results in cumulative losses of 50-60%. Recent reports indicate that this disease is becoming more severe with devastating losses of nursery fish after stocking in pre-grow out cages (Note that in Graph 2, T. mar is still a concern with isolates collected in 2009).
Graph 2. Strain collection data base*; Barramundi specific T. maritimum bacterial isolates since 2000.
Picture 10: Late stage progressive ‘saddleback' skin lesions due to T.maritimum

Picture 11: Isolation of the rod shaped bacterium on selective media illustrating the rusty colouration

Presumptive diagnosis on the farm is possible using a gram stained wet mount of a skin scrape from the edge of an infected lesion. However, before confirming T. mar as the primary reason for the lesions it is important to first rule out other possible causes such as parasites or any possible trauma history. T. maritimum is a difficult bacterium to isolate due to the requirement for a selective media and the need to sample fish at a very early stage in the progression of the disease before other more common environmental bacteria mask the isolation of the initial pathogen. Isolated colonies on selective agar media display a rusty coloured branching morphology illustrated in Picture 11.
The characteristics of T. maritimum with infections starting on the fish surface (seen clearly in Picture 10) coupled with rapid onset and transmission make therapeutic control extremely difficult. Prevention of the disease through vaccination prior to exposure is the strategy for the near future.
To date, the most important bacterial species affecting the culture of barramundi throughout the tropics is Streptococcus iniae. Indeed, S. iniae is considered to be one of the most serious bacterial diseases of all warm water fish. The R&D team has isolated S. iniae from barramundi, groupers, pomfret (Pampus argenteus), red sea bream (Pagrus major), snappers, croakers (Pseudosciaena sp.) and threadfin (Polynemidae sp.).
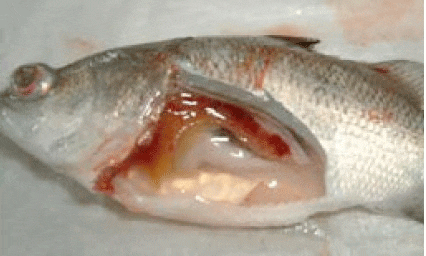
Picture 12: Typical clinical signs associated with S. iniae of exophthalmia and internal septicaemia
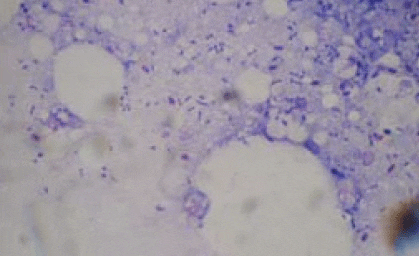
Picture 13: A gram stained, impression print of S. iniae infected brain tissue illustrating the gram-positive chains of cocci
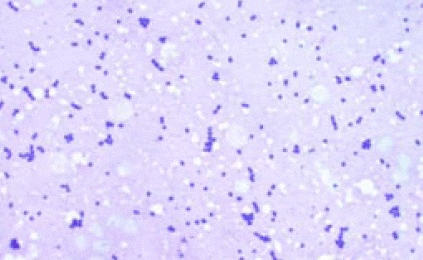
S. iniae is a gram-positive bacterium, the cocci of which group together in pairs or chains and may be clearly seen from impression prints of infected brain tissue as in picture 13. In barramundi, the disease is systemic and characterised by massive acute mortality peaks with cumulative mortality of 70% being very common. Infections can occur throughout the entire growth cycle but are more often seen in large and harvest size fish. S. iniae is therefore considered to be an ‘expensive' disease for farmers who will experience a loss of production efficiency and marketable product but more importantly a reduction in overall feed conversion rates.
The disease is associated with typical clinical signs of uni or bilateral exophthalmia (‘pop eye'), swirling swimming behaviour and scale lesions. Commonly, internal clinical signs may include cerebral meninges (brain necrosis), septicaemia, ascites (accumulation of fluid) in the body cavity and splenomegaly (enlarged spleen) as shown in Picture 12.
Since 2005, Intervet/Schering-Plough Animal Health has Norvax® Strep Si, a water-based inactivated vaccine, registered for use against S. iniae infections in farmed fish. The vaccine was designed with two applications in mind; the first of which is as an immersion vaccination in which fish are immersed for 30 seconds at approximately 2g. In the laboratory, protection from immersion vaccination has been confirmed for 5 weeks. The second application method is by intraperitoneal injection (IP) and is conducted when fish reach 15g. Laboratory studies indicate that when 15g barramundi are vaccinated using a single intraperitoneal (IP) injection with 0.1ml of Norvax® Strep Si, maximum protection is achieved as soon as one week after vaccination.
Studies conducted over 12 weeks in sterile laboratory conditions have demonstrated protection remains throughout this period. Studies under field conditions after IP vaccination at 15g have demonstrated protection up to 18 months post vaccination when fish are >2kg.
When a farming operation confirms S. iniae as a problem this should initiate a large-scale commercial vaccination program, consequently farmer profitability improves through an almost complete eradication of S. iniae outbreaks. A vaccination program of this nature typically involves the implementation of strict sanitary measures in the hatchery/nursery to ensure 100% vaccination of healthy juveniles.
Immersion vaccination at 2g in the nursery will afford protection against S. iniae until IP injection vaccination at 15g (Picture 14); this then protects the fish for the final grow out period until harvest.
Recently however, some farmers have phased out the early immersion application and reports have emerged of occasional S. iniae outbreaks in unvaccinated nursery size fish.
Picture 14: IP vaccination of barramundi.

Graph 3: Regional isolate bank of S. iniae and T. maritimum in barramundi*
This means the disease is still very much present in the farming environment and that farmers should remain vigilant and continue with the original strategy of immersion vaccination followed by an injection booster.
Conclusions Extensive mortality investigations in South East Asia by Intervet/Schering-Plough Animal Health and other research groups have resulted in a clear impression of the most important diseases of commercially farmed barramundi. Those diseases, briefly summarised in this article, include a range of parasitic, viral and bacterial pathogens.
Understanding the problem is always the first step in dealing with it and unfortunately, for most farms in the region, veterinary and on farm diagnostic support is still lacking. Even so, vaccination with Norvax® Strep Si is a commercially viable preventative strategy for the most economically devastating bacterial disease S. iniae.
When an operation decides to embark on a vaccination program it is important to consider that this is just one tool to be used in combination with a series of good husbandry and stress reduction practices. If the farming environment is poor, the vaccine might still be effective but other diseases will undoubtedly occur. Vaccines are also very specific tools and cross protection is rare, a vaccine against S. iniae for example will protect against S. iniae infections only and not against S. agalactiae.
Iridovirus and Tenacibaculum maritimum are two important pathogens of barramundi that still require effective health management strategies.

