Disease problems in shrimp production are complex and often still poorly understood. Regulations, consumer demands and sustainable management strategies restrict the number of drugs available to treat pathogens. Vaccines are likely to be ineffective in crustaceans, which lack a specific immune system similar to that of vertebrates.
Therefore, shrimp producers must consider the seed stock quality, husbandry procedures and healthy nutrition as the major tools to control disease.
Diseases are number one threat
The production of crustaceans has shown an average annual growth rate of 18% over the period 1970-2008, which by far exceeds growth for all other aquaculture species (FAO, 2010). World shrimp aquaculture is producing now well over 4 million MT of shrimp (Valderrama, 2011). This rapid increase in crustacean production largely reflects the dramatic increase in white leg shrimp culture in China, Thailand, Vietnam and Indonesia since 2000. Despite this apparent success story in terms of production expansion, shrimp production in many regions continues to suffer important economic losses due to the impact of a wide variety of diseases.
Recent events illustrate the impact of disease outbreaks on shrimp production in major producing countries. The white spot syndrome virus (WSSV), one of the main causes of the stagnating shrimp industry in the nineties, is significantly affecting shrimp production in recent years in Mexico and Brazil. Early Mortality Syndrome or Acute Hepatopancreatic Necrosis Disease (EMS/AHPND), is presently disrupting production in the three major shrimp producing countries China, Thailand and Vietnam. EMS was first reported in China in 2009, it has spread to Vietnam, Malaysia and Thailand, and now causes annual losses of billions of USD.
EMS outbreaks typically occur within the first 30 days after stocking a newly prepared shrimp pond, and mortality can exceed 70%. EMS is caused by a bacterial agent, which is transmitted orally, colonizes the shrimp gastrointestinal tract and produces a toxin that causes tissue destruction and dysfunction of the shrimp digestive organ known as the hepatopancreas. The EMS/AHPND pathogen consist of a number of unique strains of a relatively common bacterium, Vibrio parahaemolyticus, which have acquired the capabilities to release the potent toxin.
Traditional approaches to boost shrimp health through the feed
A traditional approach to reduce the impact of shrimp diseases consists of increasing the level of key nutrients affecting the health and immunology of shrimp, including vitamin C and E, phospholipids, essential fatty acids, trace minerals and carotenoids.
These “booster feeds” are often supplemented with immunostimulants, mostly derived from the cell envelope of micro-organisms, such as polysaccharides, lipoproteins, and lipopolysaccharides. The continuous use of immunostimulants is generally discouraged due to the risks for over-stimulation of the immune defense system.
Alternating on/off regimes for feed additives is often impractical in farm operations. Encouraging results to improve disease resistance have been obtained by the continuous use of health enhancing booster feeds based on the selection of the appropriate immunostimulants in combination with a balanced nutritional supply of key nutrients to support the enhancement of the immune system (Table 1).
However, the efficacy of various commercially available immunostimulants to improve stress and/or disease resistance of fish and shrimp strongly depends on the type of the product and on the supply of adjuvant nutrients that are essential to support the buildup of the immune system.
Table 1: Effect of booster feed on production parameters in a farm in NE Brazil during episode of increased disease incidence due to a combination of intensive rains and increased incidence of infectious myonecrosis virus (IMNV) and necrotising hepatopancreatitis (NHP). Booster feed based on enhanced nutritional specifications and supplementation of an immunomodulator (AQUASTIM S, Nutriad) versus standard feed.
Novel approaches (1): boosting the nutritional status and lipid reserves of the hepatopancreas
Shrimps do not tolerate high levels of dietary fat very well. A number of studies show reduced growth at levels above 10% of dietary lipid. Nevertheless, quality and quantity of dietary lipids play a primordial role in growth and health of shrimp. Shrimp have no or very limited capacity to biosynthesize a number of lipid molecules which are essential for normal growth, including cholesterol, highly unsaturated fatty acids and phospholipids. Fishmeal and fish oil are often the most important sources of cholesterol and HUFA in the diet. Increasing cost of these marine ingredients has forced formulators to reduce dietary specifications for these essential lipids.
Although these nutrient levels may not show significant differences on growth performance in feeding trials under controlled conditions, they may become critical for maintaining health and immune defenses under disease challenges and fluctuating ambient conditions encountered in production.
Furthermore, the energy status of shrimp is largely determined by its lipid reserves deposited in the hepatopancreas which functions both as a digestive gland as well as a storage depot for energy.
Therefore, farmers routinely look at squash preparates to evaluate the nutritional status of the hepatopancreas, with ample lipid reserves being an indicator of better resistance to stress and disease challenges.
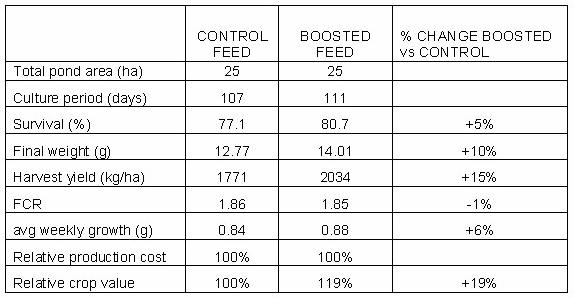
Fig. 2: The hepatopancreas is the main organ of the shrimp’s digestive system responsible for digestion, absorption and storage of nutrients. Esophagus (E), gastric mill (GM), hepatopancreas (HP), mid gut (MG), hind gut (HG), and anus (A).
Lipid digestion in shrimp occurs for a big proportion intracellular in the hepatopancreas epithelium from where it is transported to the target organs via the haemolymphe under the form of lipoproteins (Fig. 2). The formation and absorption of lipid micelles from the lumen of the hepatopancreas tubuli is therefore a limiting step in the lipid digestive process.
Digestibility enhancers based on natural emulsifying agents, selected for their compatibility with the shrimp’s digestive system, have shown to be capable of complementing the process of emulsification and absorption of dietary fats in the hepatopancreas (Coutteau et al., 2012). This in turn improves the efficiency of shrimp to use fats as essential components and as source of energy for growth and surviving episodes of stress or disease pressure.
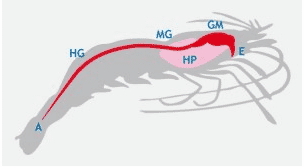
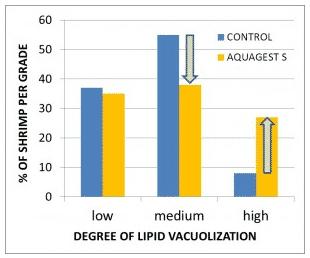
The enhancement of the lipid reserves in the hepatopancreas of white shrimp Penaeus indicus as a result of the supplementation of a digestibility enhancing additive was demonstrated recently by van de Braak et al. (2012). Histological analyses showed a three-fold increase of the percentage of shrimp with a high degree of lipid vacuolization in the hepatopancreas after supplementing the feed additive during one month (Fig. 3, 4).
The results of a parallel pond study indicated 2% higher average body weight (ABW), 4% higher survival, and 6% higher biomass for the treatment ponds. However, removal of outliers for survival from control and treatment set showed 8% higher ABW, 12% higher survival, and 23% higher biomass.
Fig. 3. Effect of the supplementation of a digestibility enhancing additive (Aquagest®S, Nutriad) on the degree of lipid vacuolization in the hepatopancreas of shrimp fed the different feeds during 30 days (van de Braak et al., 2012).
Fig. 4: Histological determination of the degree of lipid vacuolization of the hepatopancreas in shrimp Penaeus indicus, showing a high (left picture) and low (right picture) level of lipid vacuolization (100x magnification; van de Braak et al., 2012)
Novel approaches (2): Quorum Sensing technology
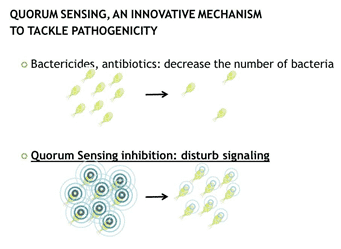
Quorum Sensing (QS) is a form of bacterial communication. Over the last decade, many bacterial species have been documented to be able to produce and secrete small signaling molecules, such as acyl homoserine lactones or certain oligopeptides, which can be detected by adjacent bacteria of the same or of distinct species. When population density raises, these molecules will accumulate in the extracellular environment, thereby providing a means for bacteria to quantitatively monitor the presence of other bacteria. These signaling molecules will, when reaching a certain threshold concentration, initiate intrabacterial signaling that culminates in the activation of specific genes. QS communication is therefore used by bacteria to synchronize gene expression alterations and coordinate biochemical responses within the entire population.
In most pathogenic bacteria from which the QS system has been studied, QS has been associated with pathogenicity, such as biofilm formation and the production of proteases, invasion factors or other virulence factors (Defoirdt, et al., 2011). In recent years, research focusing on ways to disturb QS signaling (also called quorum quenching) is therefore gaining particular interest (Fig. 5). This is especially true in the field of human medicine, where QS-inhibitors are investigated as potential alternatives to antibiotics in tackling pathogenic bacterial infections (Sintim et al., 2010).
Interestingly, chances that bacteria build up resistance against QS disruptors are predicted to be low, giving that the selective pressure against these in se non-lethal molecules is limited. This stands in stark contrast with what is seen with conventional antibiotics (Defoirdt et al., 2010).
Fig. 5: Quorum Sensing (QS), an innovative mechanism to tackle pathogenicity
Initial studies of quorum sensing in aquaculture organisms are very limited but point out exciting results. Halogenated furanones isolated from red marine algae, for example, have been demonstrated to reduce QS-regulated gene expression in Vibrio and to protect fish and shrimp from vibriosis (Rasch et al., 2004; Defoirdt et al., 2006).
At the Nutriad Technology Center, QS technology is being applied in a novel generation of natural feed additives capable of modulating gut micro flora. Compounds are tested for their capacity to inhibit QS-signaling using an array of genetically modified bacterial biosensors and QS-dependent infection protocols in simple model organisms. Using these sensitive assays, potent QS modulators, able to shut down QS signaling at concentrations far below the minimum inhibitory concentration, are being identified.
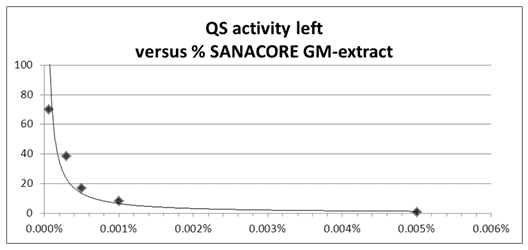
Different QS quenching activities are selected for agriculture and aquaculture species based on screening work using specific bacterial biosensors and model organisms. Synergistic blends of different natural compounds resulted to be extremely efficient in QS quenching activity against Vibrio harveyi signaling (Fig. 6).
Fig. 6: Dose – response of a synergistic blend of botanical compounds (SANACORE GM, Nutriad) on Quorum Sensing signaling activity of Vibrio harveyi. Graphs show signaling activity in QS biosensor system Vibrio harveyi BB170, relative to control, exposed to different dilutions of the product extract (Nutriad Technology Center, inhouse results).
Putting QS inhibition into practice: effect of optimizing gut health on productivity and economics of semi-intensive shrimp farming
Shrimp are actively “grazing” on the substrate present in the pond bottom and water column, and therefore highly exposed to exchanges of microflora between the environment and the digestive system. This increases the risk for the proliferation of an unfavorable gut microflora or frequent destabilization of the microflora, which can affect the optimal functioning of the digestive system.
Furthermore, the digestive system of shrimp is the main entry port for bacterial and viral infections, which remain a major risk for the profitability of shrimp production.
Sustainable approaches to modulate the gut microflora in farmed animals include the use of selected bacteria to inoculate the gut (probiotics), specific nutrients promoting the development of selected bacterial strains (prebiotics), and specific natural compounds (mostly derived from yeast and herbal extracts, so called “phytobiotics”) capable of modulating the microflora towards a favorable composition, favoring the development of beneficial bacteria and inhibiting potentially pathogenic micro-organisms. The latter strategies have the advantage of being easily applicable at the feedmill on large volumes of feed and avoiding major adaptations of the production protocols at the farm.
A synergistic blend of botanical extracts (Sanacore® GM, Nutriad) was originally selected for its bacteriostatic and bactericidal properties against pathogenic and potentially pathogenic bacteria in vitro using the disk diffusion method. Furthermore, this synergistic blend has proven to be a powerful interrupter of bacterial QS signaling at concentrations well below minimal inhibitory concentrations (see above), allowing it to effectively modulate the gut flora towards a more favorable composition.
The supplementation of Sanacore GM promoted growth significantly in healthy shrimp growing under controlled lab conditions; showing a remarkable 20% increase of weekly weight gain and 4% improvement on food conversion (Coutteau et al., 2010). The effect of this botanical product showing combined activities in QS inhibition and bactericidal action against a wide range of pathogenic bacteria was verified in a semi-intensive shrimp farm in Panama.
The second production season in Panama, stocked in aug-sept, is characterized by unstable climatological conditions, resulting in strong temperature fluctuations which in turn affect shrimp growth and increase the impact of outbreaks of white spot virus (WSSV).
The first production cycle, seeded in jan-april, provides more suitable growth conditions and generally results in better survival and productivity. Two treatments were compared which only differed with regard to the supplementation or not of the phytobiotic growth promoter (Sanacore® GM) to the standard feed used at the farm.
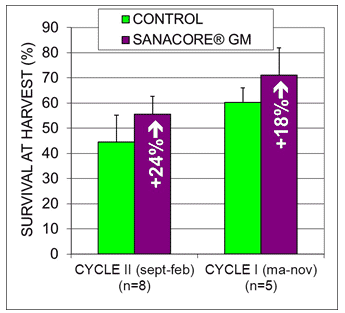
The supplementation of the botanical feed additive drastically improved survival, amounting to a relative increase with 24% and 18% compared to the control group for the dry and wet cycle, respectively (Fig. 7). Natural White Spot Disease outbreaks were observed during shrimp farming in both treatments under similar frequency and severity; WSSV virus was confirmed by immuno-chromatography and nested-PCR tests.
The presence of a synergistic blend of phytobiotics provided an array of antimicrobial activities in the shrimp’s digestive system. This offered additional protection against co-infections with opportunistic bacteria such as vibriosis, often the major cause of mortality in WSSV-infected shrimp (Phuoc et al., 2009).
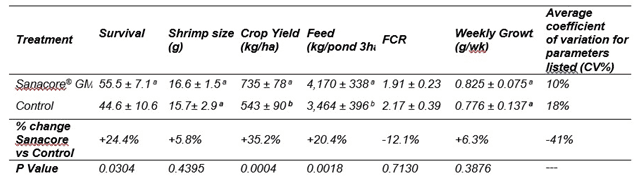
The evaluation in the second cycle on 8 replicates per treatment allowed a good evaluation of variability among ponds for the different production parameters. The addition of the phytobiotic reduced drastically the variability of production results among ponds fed the same feed (average coefficient of variation between ponds for the 6 production parameters : control 18% versus Sanacore group 10%; Table 2). This further indicated the importance of increased control of gut microflora on the reproducibility of production in semi-intensive pond environments.
Fig. 7: Survival percentage at harvest for control ponds and treatment ponds receiving the phytobiotic supplement in two production cycles in semi-intensive production of white shrimp L. vannamei (average and standard deviation of 8 and 5 replicate ponds of 3ha per treatment, respectively; data from Vaca et al., 2010, 2011).
Table 2: Production results for P. vannamei in Panama during the second production cycle for control ponds and treatment ponds receiving a phytobiotic supplement after 141 days of culture (average and standard deviation of 8 replicate ponds of 3 ha per treatment).
March 2015