The disease has been estimated to cost the UK trout industry approximately £2.5 million per year and is one of the main research topics in the Aquatic Vaccine Unit at Stirling. Both short and longterm approaches are being taken to tackle this disease, including the testing of novel treatments, elucidation of the life cycle, development of a vaccine to prevent PKD, and selective breeding of PKD-tolerant fish.
Infectivity trials demonstrated that a single T. bryosalmonae spore released from a bryozoan (invertebrate intermediate host) can infect a rainbow trout resulting in it developing clinical PKD. While previous studies have indicated that rainbow trout in the UK are likely to be aberrant hosts to the parasite, we have recently demonstrated that brown trout are true hosts, with the parasite being able to cycle between bryozoans and this fish species. Increased knowledge of the life cycle of T. bryosalmonae and the ability to keep the parasite in the laboratory is a significant step forward in allowing us to understand PKD.
Current work at Stirling, funded by the BBSRC (Dr Morris, Prof. Adams) is focused on charting the development of T. bryosalmonaein both the bryozoan and salmonid host and how environmental factors affect spore production/ release. In a related project jointly funded by DEFRA/Schering Plough Aquaculture (Dr McGurk, Dr Morris, Prof. Adams) recombinant vaccine is being developed to protect fish against this important disease. Another approach is being taken in a third project funded by Defra and the British Trout Association to investigate the possibility of breeding PKD resistant fish (Garreth Butterfield PhD student; Prof. Brendan McAndrew/ Dr Morris/Prof. Adams). Our progress in these projects is explained below.
Rainbow trout are collateral casualties in the life cycle of Tetracapsuloides bryosalmonae
 |
| A prime rainbow trout |
PKD is a severe economic constraint on the rainbow trout industry in Western Europe, usually resulting in 100% morbidity of newly introduced fish on affected farms. The parasite infects the kidney of the fish where it replicates causing a severe inflammatory response. This puts enormous strain on the fishes renal and immune systems making them very susceptible to additional stressors. While 20% mortalities associated with PKD are common, losses over 80% have been reported. Research in Europe suggests that the disease is having serious effects on the stocking of wild salmonids while in the UK increasing numbers of salmon hatcheries are reporting outbreaks.
Initial studies on T. bryosalmonae demonstrated a distinct seasonality of PKD where it only appeared on farms in the summer months, the rainbow trout that survived an outbreak developing a resistance to further exposure. Attempts at transmitting the parasite from fish to fish failed suggesting that another host was involved in its life-cycle. Studies on related myxozoan parasites had demonstrated that an oligochaete worm was often required in their life-cycles and therefore thousands of these worms were examined to determine whether they might be infected with T. bryosalmonae. However, not one of these worms was positive and the prospect of the finding the host looked as if it may require screening all of the invertebrates in a river system. By serendipity a group examining freshwater bryozoans (colonial, sessile, filter-feeding invertebrates) at Reading University noted an unusual parasite infecting them. 18ssu rDNA sequencing of this parasite determined that it was T. bryosalmonae and subsequent transmission studies demonstrated that rainbow trout could be infected when exposed to infected bryozoans. While bryozoa were found to harbour the parasite, the life-cycle of T. bryosalmonae was still not complete with the important question of how the bryozoans became infected in the first place and the role of fish needing to be resolved.
In 2004 a three-year grant funded by the Biological and Biotechnology Research Council (BBSRC) was awarded to Dr. D.J. Morris and Professor A. Adams at the Institute of Aquaculture to complete the life-cycle, document the development of T. bryosalmonae spores and examine environmental factors influencing the release of these spores.
To conduct the research a custom built facility was set up to grow bryozoans in and used for the transmission experiments (Figure 1). The bryozoans within this system were fed a custom mixture of cultivated algae and protozoa developed at Stirling. Unlike previous attempts at fish to bryozoa transmission, the trials used a salmonid species in which T. bryosalmonae spores were known to develop (brown trout Salmo trutta) and the exposure timed to when the fish were known to be releasing these spores into the environment. These experiments resulted in the successful transmission of the parasite to bryozoans which were then used to infect naïve brown trout thus both completing the life-cycle and demonstrating that the parasite could cycle between these two hosts indefinitely.
 |
| Bryozoan cultivation tanks and transmission trial unit |
The fact that rainbow trout produce an exaggerated immune response during PKD and that T. bryosalmonae spores have never been reported within this species in the UK strongly suggests that it is an aberrant host. This is in contrast to rainbow trout in the USA where T. bryosalmonae spores have been reported, and where PKD is not usually considered a major problem. This suggests that there are at least two strains of T. bryosalmonae; a North American one adapted to Oncorhynchus spp. and a European one adapted to Salmo spp. Therefore, O. mykiss introduced into European waters are likely to be infected with the wrong strain of the parasite which results in the severe host response and subsequent disease.
Our studies into the development of T. bryosalmonae within bryozoans have demonstrated higher water temperatures (18°C) result in the parasite uncontrollably replicating, producing thousands of spores until the host is killed. During the experiments we also discovered that infected bryozoans can fragment forming new colonies harbouring the parasite which are capable of spreading and establishing themselves away from the original colony.
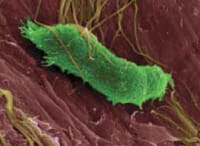 |
| Presaccular stage of Tetracapsuloides bryosalmonae within the bryozoan host Fredericella sultana |
The resolution of the life-cycle of T. bryosalmonae and the development of culture methods for the bryozoan host have finally allowed us to create a ‘parasite factory’ within the laboratory. This facility will be invaluable for future studies looking at controlling PKD in farmed salmonids.
PKD- treatments and vaccines.
As with many other parasitic diseases, there are currently no licensed prevention or treatment protocols for proliferative kidney disease (PKD). Previously, the use of malachite green mitigated the severity of PKD but its withdrawal has led to farmers having to employ alternative management systems - such as carefully timed exposure programmes - with limited success. Studies conducted at the Institute of Aquaculture examining the fungicidal agent fumagillin demonstrated that this compound and its analogues were efficacious in reducing parasite numbers within infected fish. However, they were also potentially damaging to the fish’s immune system and were therefore unlikely to be approved for use.
A recent NERC/Schering-Plough Aquaculture/ Fishmongers’ Co. funded research project within the Aquatic Vaccine Unit focused upon the laboratory culture of bryozoans alongside the further examination of chemical control methods. Using the cultured bryozoans it was possible to conduct an in-depth light microscopical study of the development of the parasite within the bryozoan host. Using confocal microscopy the three dimensional structure of the infective spore was also determined revealing an elegant architecture. Infectivity trials established that exposure to a single spore of T. bryosalmonae was sufficient to lead to full-blown PKD in rainbow trout. This explains the high morbidity levels witnessed on farms as even relatively small bryozoan colonies have the potential to produce thousands of spores when infected. Drug trials were conducted using in-feed medications but none were found to be sufficiently effective to justify commercial refinement.
The knowledge that those fish that recover from clinical disease can display resistance to future challenge with T. bryosalmonae is crucially important for the development of a prospective vaccine.
A vaccine development project is currently being funded by Defra and Schering-Plough Aquaculture within the Institute of Aquaculture. It aims to overcome some of the shortcomings encountered using in-feed preparations of chemotherapeutants. Importantly, a protective component of the parasite has been identified and purified. Using its DNA sequence, large quantities could be produced and used as an antigen for the vaccination of fish. This prospective vaccine would play a key role in the control of PKD both on farms and limiting the spread in wild populations of salmonids.
The first Proliferative Kidney Disease tolerant rainbow trout?
Selective breeding has been utilised for many years in the Atlantic salmon industry, and the results from such schemes have been widely documented. Benefits, such as increased growth rate, reduced food conversion ratio (FCR), improved survival and disease resistance are now traits that salmon breeding companies use to improve the performance of the offspring in each generation. However, in rainbow trout no such selectivity has been utilised and projects to assist farmers in reducing costs and, in turn, improve margins, have long been needed. Finally, the trout industry is beginning to utilise the application of selective breeding on strains currently used commercially.
A few years ago, a LINK Aquaculture project on the application of selective breeding to UK rainbow trout took place. The project was a collaboration between the Institute of Aquaculture, the Roslin Institute and the British Trout Association. Together they conducted a survey to identify what farmers thought to be the most important traits relating to commercial production. The general response included, as expected, growth rate, improved FCR, harvesting yield, flesh colour etc. But when asked to identify significant diseases, Proliferative Kidney Disease (PKD) was repeatedly mentioned, especially by farmers located in Southern England, where outbreaks of the disease are renowned to cause mass mortality, or, at best massive financial burdens.
The current project involves two farms, who provided broodstock to produce 540 genetically related families (Farm A: two strains – 160 families, Farm B: one strain – 380 families). The individuals were reared communally under commercial conditions and their performance compared. During the growing period there was a severe outbreak of PKD, creating a natural challenge, all families being equally exposed to the causative agent, T. bryosalmonae. At the peak of the infection in September 2004 a sample of 1500 fish was removed and analysed for disease severity using a kidney swelling score (scale 0 – 4; 0 showing no signs of infection, 4 severe infection) and growth parameters. The individuals were identified to the family level using microsatellite markers. The additive genetic variation was calculated and generated significant heritabilities (higher the better).
Farm A = 0.34±0.08 (±Standard Error)
Farm B = 0.25±0.06 (±Standard Error)
The next stage was to identify the most and least tolerant strains/families by calculating Estimated Breeding Values (EBVs) in kidney units. (Lower = better resistance).
Farm A = Min 1.69, Max 2.95, Average 2.33
Farm B = Min 1.54, Max 2.83, Average 2.17
The data also expressed a significant negative correlation of -0.4 between length and weight, and kidney score, suggesting bigger fish appear to be more resistant to the disease. A simple explanation of this correlation would be; healthier fish continue to feed and grow, whilst affected fish become stressed and lose appetite.
To confirm the calculated values, the siblings of those fish taken for analysis or the original parents (all of which have known EBVs) have been used as breeders to create high and low tolerance families that are currently being assessed under controlled challenge experimental conditions at the Institute of Aquaculture. The controlled challenge will enable the magnitude of the resistance to be measured and, possibly, the resistance mechanism to be determined. The knowledge gained will allow future selections to be made at the farm level that will inevitably improve the tolerance of stocks on sites where PKD is prevalent.
The benefits of undertaking a study of this magnitude and to this level of certainty will not only aid the farms currently involved, but will eventually be a benchmark for the rest of the industry. Selective breeding is a useful tool that should, and will inevitably, be utilised by the entire British rainbow trout farming industry. The advantages can be visualised easily, simply by referring to the genetic gain that has been achieved in other fish species.
Footnote
- This article was originally published in Aquaculture News, produced by the Institute of Aquaculture, University of Stirling.
Further Reading
| - | View the contents of the Finfish Winter/Spring 2008 Publication by clicking here. |
Winter/Spring 2008



