Shrimp farming in Bangladesh has expanded rapidly since the 1980s. The main species cultured is black tiger shrimp (Penaeus monodon), known as bagda in Bengali. At present, the area under tiger shrimp production is 188,046 ha, of which around 5% is located in the district of Cox's Bazar in the southeast of the country, and the remaining 95% in the districts of Khulna, Bagerhat and Satkhira in the southwest ( Belton et al., 2011). Bangladesh produced 56,569 MT of tiger shrimp and 39,868 MT giant freshwater prawn (Macrobrachium rosenbergii) in 2010–2011 with an export value of approximately $462 million ( DOF, 2012). The sector is the country's second largest source of export earnings after readymade garments, and shrimp farms alone directly employ in excess of 600,000 people ( Karim et al., 2011 and USAID, 2006). The majority of shrimp farms in the coastal region of Bangladesh follow extensive culture practices, relying mainly on natural productivity, with limited or no management in respect of drying and plowing the gher 1 bottom between crops, or liming, fertilization and feeding. The stocking density for PL typically ranges from 0.2 to 1.5 PL/m2 and annual yields of shrimp are low; in the order of 160–230 kg/ha ( Belton et al., 2011).
This low level of intensity is in part a risk mitigating strategy deployed by farmers in response to the pervasive and highly destructive White Spot Disease (WSD) (Karim et al., 2011). WSD, which is caused by White Spot Syndrome Virus (WSSV), is capable of causing 100% mortality within a few days of the onset of clinical signs (Ayub et al., 2008, Sanchez-Martinez et al., 2007 and Vaseeharan et al., 2003). Until the recent emergence of EMS (Leano and Mohan, 2012), WSSV was considered the most serious disease problem for shrimp aquaculture in Asia (Hossain et al., 2001 and Otta et al., 2003), where it over-shadowed ‘all other disease agents as the leading cause of production losses’ (Kanchanaphum et al., 1998, p1).
In Bangladesh, WSD first occurred in cultured tiger shrimp in semi-intensive farms in Cox's Bazar in 1994, resulting in their permanent closure. In 1996 the disease spread to Khulna region in the southwest of the country, affecting approximately 90% of extensive shrimp farms and causing a 20% drop in national shrimp production. Shrimp exports fell from 25,742 tonnes to 18,630 tonnes in 1997–1998. They subsequently rebounded in 1999–2000, only to fall by 25% in 2001 as production was again badly affected by WSSV associated with other viral and bacterial pathogens (Alam et al., 2007). WSD is now endemic and is generally regarded as one of the most important constraints to the industry's sustainability and further expansion in Bangladesh (Karim et al., 2011). As a result, finding ways to minimize the incidence of WSD could have significant implications for the future success of the industry.
The first shrimp hatchery in Bangladesh was established by the Department of Fisheries (DOF) at Cox's Bazar in 1987. At present, 57 shrimp hatcheries are in operation, all of which are located in Cox's Bazar (BFFEA, 2009). Present annual demand of bagda PL, as reported by the secretary of the Shrimp Hatchery Association of Bangladesh (SHAB), is about 8 billion and present annual production is about 6–7 billion, where hatcheries have the capacity to produce 15 billion PL (Pers. Comm. Homayion, 2010). The gap between production and demand is met by harvest of wild PL.
Domesticated P. monodon broods are unavailable in Bangladesh and hatcheries are completely dependent on wild broods captured from the Bay of Bengal. However, unlike domesticated broodstock, which can be raised in a controlled, disease free environment, wild broodstock are frequently exposed to or infected by pathogens, including WSSV. WSSV can be vertically transmitted from WSSV-positive spawners to their offspring ( Lo et al., 1996b). This has important implications for the prevention of disease at the growout stage since infected postlarvae can represent a major source of infection for shrimp farms ( Sanchez-Martinez et al., 2007). This means that screening and selecting WSSV-negative brooders can markedly reduce the chances of a subsequent outbreak of WSSV ( Vaseeharan et al., 2003), providing that appropriate management practices for reducing the likelihood of horizontal transmission during are also followed ( Karim et al., 2011).
The presence of WSSV in wild caught P. monodon broods taken from hatcheries has been confirmed by a number of studies. Ayub et al. (2008) reported infection rates of 20% and 30% from samples of 60 spawners taken from hatcheries in Cox's Bazar district, using non-nested and more sensitive nested PCR testing techniques respectively. Research by Vaseeharan et al. (2003) revealed that 34% of P. monodon brooders taken from hatcheries on the east coast of India tested positive for WSSV by PCR, while Hossain et al. (2001) recorded the incidence of WSSV observed in Indian tiger shrimp broodstock at 50%, and Remany et al. (2012) found the prevalence of WSSV infection in tiger shrimp broods captured off the southeast coast of India to be 21%.
Lo et al. (1996b) also reported that approximately 48% of captured P. monodon broodstock tested in Taiwan over an eight month period were WSSV-positive, but that prevalence varied considerably with season. In another longitudinal study, Iqbal et al. (2011) found the incidence of WSSV in broods from hatcheries in Cox's Bazar to range from 0% in September to as high as 90% in May/June, with a similar, but delayed, pattern of temporal variation evident in nauplius and postlarvae. Withyachumnarnkul et al.'s detailed study from Thailand also revealed seasonal fluctuations in the percentage of WSSV-positive prevalence in P. monodon broods of between 0 and 18%, which were matched by a highly significantly correlated (p ? 0.01) pattern of peaks and troughs in prevalence in PL occurring approximately 1 month after those for the broodstock ( Withyachumnarnkul et al., 2003). 2
The studies cited above clearly demonstrate the presence of WSSV in wild P. monodon broods used in many hatcheries, along with the likelihood of vertical transmission to the PL. However, none of them attempt to link the prevalence of infection to the origin of broods, with the exception of Vaseeharan et al. (2003) which only does so as far as the hatchery itself, and not to the fishery from which the spawners were captured.
One of the authors of this paper, working in the shrimp hatchery sector in Cox's Bazar for a number of years, has observed the commonly held perception among hatchery technicians that broods captured from deep zones of the Bay of Bengal are affected by WSSV less frequently than those from shallow zones. Discussion with owners of fishing vessels confirmed that they caught shrimp broods mainly from two separate depth zones; a deep zone and a shallow zone, where depths ranged from 40 to 50 m and 15 to 25 m respectively.
This paper attempts to evaluate the accuracy of the observation that P. monodon spawners harvested from shallow zones of the Bay of Bengal are more likely to test WSSV positive than those captured from deeper zones, and explores interactions between this factor and the seasonal variations in WSSV prevalence noted above. In order to achieve this, the present study sampled broods captured from shallow and deep zones of the Bay of Bengal from five hatcheries in Cox's Bazar over a six month period. WSSV was tested for using Polymerase Chain Reaction (PCR) testing facilities. Length, weight, fecundity and hatching success were also measured in order to check for any association with brood origin, or correlation with WSSV.
The paper also explores the possible implications of these findings for the shrimp sector, taking into account a number of social and economic features of hatcheries and brood supply networks which influence the likelihood of any corrective recommendations or policies being successfully implemented. In addition to quantitative data pertaining to shrimp broods, the Results section therefore also provides qualitative information regarding the characteristics of the brood fishery which are important to this analysis.
Results
Relationship between size and reproductive performance
Descriptive statistics on the mean monthly weight, length, fecundity and hatching rate of broods from shallow and deep zones are presented in Table 1. The maximum and minimum values for each variable are indicated in the table. Average weight across the sample period was 93.17 ± 12.73 g and 152.30 ± 23.11 g for broods collected from shallow and deep zones respectively. Considering both regions, the average weight of broods was 122.73 ± 35.00 g. Average length over the sample period was 20.92 ± 1.301 cm and 26.77 ± 1.751 cm for broods collected from shallow and deep zones respectively. The average length of all broods collected from the two regions was 23.84 ± 3.315 cm. Average fecundity for the period in which the research took place was 0.34 ± 0.058 million and 0.65 ± 0.090 million eggs for broods from shallow and deep zones respectively, and 0.50 ± 0.170 million considering broods collected from both regions. Average hatching rate over the survey period was 74.34 ± 6.92% and 88.99 ± 5.08% respectively for broods collected from shallow and deep zones, and 81.67 ± 9.52% overall.
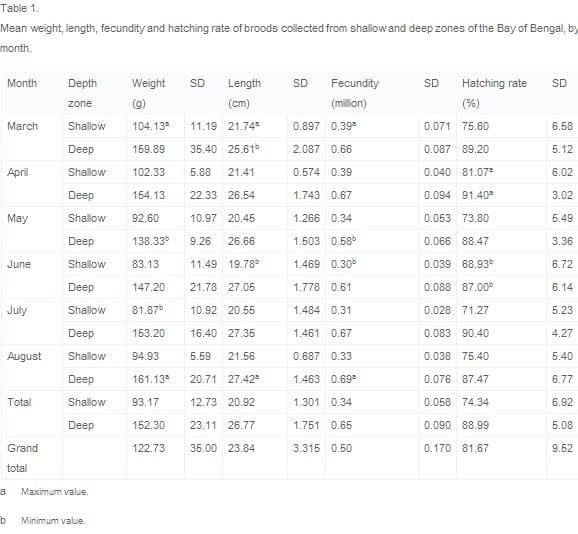
Fig. 1 and Fig. 2 present data on monthly average size and monthly average reproductive performance for all broods. Length/weight and fecundity/hatching rate are closely related. Similarities are apparent in the shape of all four curves, both of which are at a maximum in March/April and at a minimum in June, suggesting that size is positively associated with reproductive performance. Fecundity as calculated in terms of eggs per gram of brood mother was between 10% (March) and 24% (August) higher in deep zone broods than in those harvested from the shallow zone.
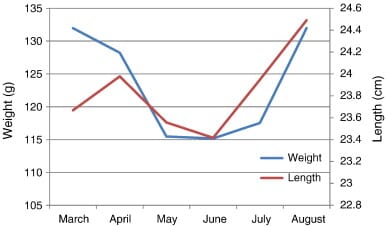
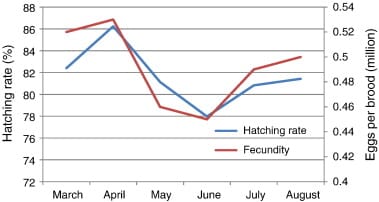
A strong positive correlation (R2 = 0.878) was found between brood weight and length. The analysis also showed a highly positive correlation between the brood weight and fecundity where the R square linear value was 0.92. Hatching rate was also significantly positively correlated (R2 = 0.654) with fecundity.
WSSV prevalence and depth zone
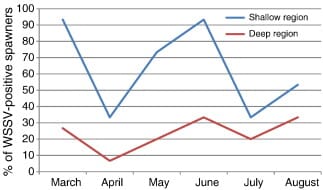
WSSV test results indicated the very poor condition of broods collected from the shallow zone. Of the 90 brood-mothers collected from the shallow zone, 57 (63.3%) were found to be WSSV positive, whereas only 21 (23.3%) of 90 collected from the deep zone were WSSV positive (Fig. 3).
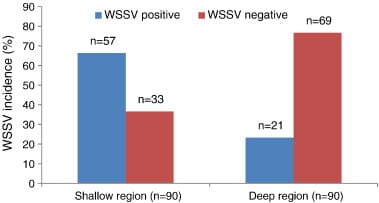
The prevalence of WSSV was found to be highest during the months of March and June, peaking at 93.3%, and lowest (33.3%) in the month of April. Among broods collected from the deep zone, highest WSSV prevalence was 33.3% in June, falling to 6.7% during the month of April (Fig. 4). This is the reverse of the pattern indicated in Fig. 1 and Fig. 2 above, suggesting a negative association between WSSV infection and size and reproductive performance.
Relationship between WSSV status, brood size and reproductive performance
Table 2 presents statistics on differences in the average length, weight, fecundity and hatching rate of WSSV negative and WSSV positive broods. This reveals that broods harvested from the deep zone were significantly larger and had significantly better reproductive performance than broods harvested from the shallow zone in all cases, irrespective of WSSV status.
Relationship between depth zone and WSSV status
Table 3 presents the same data, reorganized to allow for comparison of the average length, weight, fecundity and hatching rate of broods from shallow and deep zones. This indicates that there was no significant difference in size (length/weight) and fecundity among WSSV negative and WSSV positive broods harvested from the shallow zone and, similarly, no significant difference in size and fecundity among WSSV negative and WSSV positive broods harvested from the shallow zone. However, WSSV negative broods were found to achieve a significantly (p = 0.000) higher hatching rate than WSSV positive broods, irrespective of whether they were harvested in shallow or deep zones.
The variables depth zone, brood size and WSSV prevalence appear inter-correlated (i.e. broods harvested from the deep zone are larger on average than broods from the shallow zone; large broods tend to have a low incidence of WSSV; incidence of WSSV infection is lower among broods harvested from the deep zone than among broods harvested from the shallow zone). However, comparison of Table 2 and Table 3 suggests that depth zone has an effect on likelihood of WSSV infection that is independent of brood size, since there is no statistically significant difference in the size of WSSV positive and negative spawners harvested from each depth zone. WSSV does have a negative effect on reproductive performance (hatching rate) however, irrespective of depth zone.
Qualitative results: brood fishery characteristics
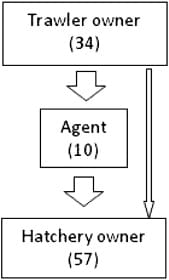
There are 36 fishing vessels which target brood shrimp operating out of the port of Chittagong in southeast Bangladesh. All broods used in hatchery operations in Bangladesh are collected by trawl fishing. Two hatcheries possess trawlers of their own which catch broods to meet their own needs and sell additional broods to other hatcheries after this demand has been fulfilled. All other broods are captured by brood trawlers without direct links to hatcheries and distributed through 10 agents who act as intermediaries between trawler owners and hatcheries (Fig. 5). Agents pay between BDT 1 and 2 million (USD 12,000–24,000) to trawler owners each year to secure the right to act as marketing intermediaries, with each agent acting as the exclusive intermediary for several boats.
Brood trawlers normally make offshore fishing trips lasting two days. Each trawler typically catches more than 100 brood mothers in a single trip during January to March, when broods are most readily available. This falls to as few as 10 individuals per trip during June and July. Brood supply is thus somewhat out of sync with demand of PLs from farmers, which begins in January in some areas and peaks between March and July.3 The difference between brood supply and PL demand is reflected in Fig. 6, which includes data on the average monthly price paid for individual brood mothers by hatcheries in Cox's Bazar.
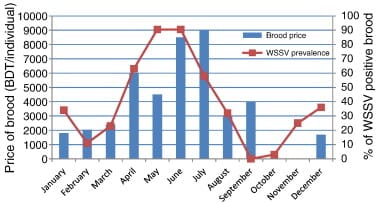
Boats returning from each fishing trip contact their agents to inform them of the number of broods captured. Agents set the price of broods for the day depending upon both the availability of broods and the level of demand from hatcheries, and inform hatchery owners of this price. On any given day there is very little variation in the price of brood shrimp (in part because only a small portion of the total fleet brood trawlers make landings each day), but prices can vary considerably from month to month and from week to week, depending on the interaction of demand and supply (Fig. 6). Hatchery owners order the number of broods they require from agents without having had the opportunity to inspect them and, having made this decision, are obliged to purchase the quantity stated.
Hatchery workers meet the ship in order to receive the broods they have ordered. When demand for broods is high, hatchery owners are obliged to accept any broods that they are offered, regardless of quality and whether or not they are gravid. They must also pay the same price per individual regardless of its condition. In broods which are not yet gravid, ovulation is induced through eyestalk ablation; a process which takes 7–8 days and incurs high mortality rates. Hatchery workers only have the option to select broods based on their perceived quality during periods of excess supply.
Discussion
According to FAO (2006) the body length and weight of healthy tiger shrimp females are 25–30 cm and 200–320 g respectively. The present study recorded an average length of 20.92 ± 1.30 cm and 26.77 ± 1.75 cm, and average weight of 93.17 ± 12.73 g and 152.30 ± 23.11 g for broods collected from shallow and deep zones respectively. The size of females in this study is thus considerably smaller on average than those reported by FAO (2006). The reasons for this are not certain, but might be indicative of stocks of broods being overfished, leading to a shortage of large mature adults. If this interpretation is correct it has serious implications for both the ecology of the Bay of Bengal and the future sustainability of the shrimp industry in Bangladesh.
The life cycle of P. monodon includes several distinct developmental stages, during which it is found in different habitats. Reproduction takes place in offshore deepwater zones with high salinities. Once hatched, larval shrimp drift toward the coastal zone where they mature into juveniles and, subsequently, adults migrate back to deeper offshore zones ( Motoh, 1984). Young adults (less than one year) tend to be found at shallower depths than older, larger adults. As a result, brood trawlers tend to collect young adults from shallower zones because of the lower costs and time involved in doing so.
It was not possible to determine why broodstock captured in the shallow zone are smaller than those in the deep zone on the basis of this study alone. It is possible that the size difference between shrimp from shallow and deep zones simply reflects the natural migration pattern of P. monodon under which young adults migrate from shallow to deeper waters, since individuals, which are already infected with WSSV, are less likely to reach the deep zone because they die during migration. However, Hoa (2009) notes that environmental parameters such as salinity, pH, temperature, ammonia and nitrite are known to be very stable and/or low in the deep sea, and that this reduces stress to adult tiger shrimp. If P. monodon remain locally resident for extended periods of time, this would suggest that the larger size of broodstock found there is a result of the optimal conditions for growth and lower chances of infection which deep waters provide.
Coyama et al. (1991) have reported variations of reproductive quality of shrimp broodstock to be primarily genetic in nature. However, the present study shows that the size of spawners also influences spawning success, being positively correlated with hatching rate. This conclusion is supported by Motoh (1981), who established a positive correlation between fecundity and brood size in terms of carapace length, and Villegas et al. (1986) who demonstrated a positive correlation between fecundity and spawner weight. Similarly, Jiang et al. (2009) found that the age and size of male P. monodon significantly influenced the formation, development and maturation of male spermatophore. The present study also demonstrates a negative relationship between WSSV infection and hatching rates.
According to FAO (2007) the average quantity of eggs produced per tiger shrimp brood is in the range of 0.2–0.4 million eggs for females of 90–150 g body weight, and up to 0.45–1.0 million eggs for 160–300 g females. In the present study, average fecundity was found to be 0.34 ± 0.058 million from the broods collected from shallow zones of the Bay of Bengal and 0.65 ± 0.090 million from the broods collected from deep zones for broods averaging between 93 g and 152 g — i.e. fecundity of the deep zone broods was about double the fecundity of shallow zone broods. Das et al. (1997) reported a mean hatching rate of 88.6% among tiger shrimp broods in India, which is higher than the mean hatching rate of 81.7% for this study, but comparable to the mean hatching rate of 89.0% for deep zone broods. Hatching rate of eggs was about 15% higher in broods collected from deep zone than the broods collected from shallow zone of the Bay of Bengal in the present study.
WSSV prevalence was very high in the broods collected from the shallow zone as compared to those broods collected from the deeper zone. On average over the six months during which samples were taken, 63.3% of broods from the shallow zone were WSSV positive, as opposed to 23.3% of broods collected from the deep zone of the Bay of Bengal. This data therefore suggests that it might be possible to reduce vertical transmission of WSSV (from mother to off-spring) by 46%, simply by the exclusive use of broods collected from deep zone of the Bay of Bengal. Data on reproductive performance presented above also suggest that the use of broods in hatcheries in Bangladesh might be reduced by about 50% if only broods originating from the deep zone were used.
However, this is unlikely to occur for a number of reasons relating to the availability of broods and the economic incentives of brood agents and hatcheries. In 2007 the Department of Fisheries introduced a law precluding all trawlers from fishing at depths of less than 40 m. This was strictly enforced during the months of April and May, but enforcement of these regulations was quickly relaxed for brood trawlers as they resulted in a crisis in broods and PL supply which threatened the output of the shrimp sector. A shortage of deep zone broods is compounded by the marketing system, in which all broods landed on any given day have a single undifferentiated market price. This situation makes it impossible for hatcheries to pay a premium for high quality broods should they wish to, and means that there is no price incentive for trawlers to attempt to target broods which are large, are already gravid, or originate from the deep zone.
This tendency is compounded by seasonality of demand for PL. As Fig. 6 shows, prevalence of WSSV in broods4 corresponds very closely with periods when demand for PL is high and supply of broods is constrained (as indicated by the prices paid for broodstock by hatcheries). This means that hatcheries will buy any broods they can obtain, regardless of quality, during times when the incidence of WSSV and its transmission to PL is at its highest.
In addition, there is a common perception among hatchery owners that WSSV is a problem which affects farmers (i.e. after infected PL have been stocked) and therefore has no bearing on the profitability of hatchery operations. However, this study indicates that eggs from WSSV infected broods have a significantly lower hatching rate than those WSSV negative spawners. In addition, it is common for hatcheries to experience high brood mortality both among gravid females before spawning takes place, as well as among those which have undergone eyestalk ablation, and it is very likely that in many cases this is related to WSSV which is expressed following the exposure to the stress of being captured and transferred to the hatchery environment. This implies that the cost to hatcheries of WSSV may actually be considerable, particularly given the high price of broods during the months of peak demand and disease incidence.
Conclusions
The ultimate reasons for the significant differences in the proportions of WSSV positive broods from shallow and deep zones of the Bay of Bengal which this study reveals are not known, although it may be linked to higher fluctuation of water quality parameters (particularly temperature and salinity) closer to shore. This interpretation seems to correlate with the spike in WSSV incidence during the monsoon season. Polluted or nutrient rich runoff and perhaps even discharges of WSSV positive water from hatcheries and shrimp farms may also play a part in encouraging greater levels of infection closer to shore. This hypothesis is supported by the results, which show that, although there is a clear association between brood size and WSSV incidence across the entire sample, there is no causal relationship between brood size and WSSV infection rates within each region (i.e. there is no significant difference in the size of WSSV positive and WSSV negative broods from either the shallow zone or in the deep zone).
What is clear from the data presented above is that, although exclusive use of deep zone broods in hatcheries might offer significant advantages, the availability and market conditions described above make such a scenario very unlikely. The high occurrence of WSSV in wild populations in shallow zones of the Bay of Bengal during periods when demand for broods is greatest also means that any future legislation aimed at mandating the use of PCR tested PLs (such as has been implemented in India and a number of other countries) may face practical difficulties with hatcheries unable to secure enough WSSV negative broods to facilitate production of sufficient quantities of WSSV negative PL to meet the needs of the entire industry. Ultimately, the only viable solution to this problem may be to develop domesticated stocks of P. monodon broods which can be certified as specific pathogen free (SPF), as is ongoing in Taiwan, Brunei, Hawaii, Thailand and India, among others. Such an effort, if attempted, would likely require considerable sustained investment, at least in its initial stages however, meaning that this option also may remain out of reach, at least for the immediate future. An interim measure may be to rely on imported SPF tiger shrimp broods, which have recently become commercially available.
February 2014



