According to Wikipedia “A monogastric digestive system works as soon as the food enters the mouth. Saliva moistens the food and begins the digestive process. After being swallowed, the food passes from the esophagus into the stomach, where stomach acid and enzymes help to break down the food. Bile salts stored in the gall bladder empty the contents of the stomach into the small intestines where most fats are broken down. The pancreas secretes enzymes and alkali to neutralize the stomach acid.”
The “stomach acid” working in all monogastric animals is hydrochloric acid, a very strong inorganic acid, which is produced by gastric glands (parietal cells). This acid is able to lower the pH in the stomach to levels between pH 1-3.
The hydrochloric acid production at birth is negligible, but it will increase while ageing of the animal. The more acid is produced in the stomach, the lower is the pH. The present pH is involved in the activation of pepsin, which is a proteolytic enzyme. This means it is needed in the digestion of protein. Pepsin is secreted as an inactive zymogen, called pepsinogen (inactive in order to not “digest” the stomach itself when no food is available), and its conversion into the active form is catalyzed by the action of the acid. Like every enzyme, pepsin has certain optimal conditions in which it is working best. The optimal pH for pepsin activity is 2.0. At higher pH-levels the activity is severely reduced. So far the theory…
What kind of implications does this have for monogastric aquaculture species, which are heavily depending on the high protein inputs – and on the proper digestion of these expensive ingredients?
One of the possible answers may come from feed additives! The use of organic acids or acid salts has been studied in numerous publications over the past half-century in animal nutrition (Cole et al., 1968). Supplementing diets with organic acids reduces the pH in the stomach, it stimulates thereby the activation of pepsinogen to pepsin and thus may improve protein digestibility and decrease the rate of gastric emptying; further improving protein digestion by increasing the rate of proteolysis of large protein molecules (Theobald and Lückstädt, 2011). The reduction of pH in the feed and stomach largely depends on the buffering capacity of feed ingredients. Animal protein (e.g. fishmeal), extensively used in aquaculture diets, has a 15 fold higher buffering capacity compared to cereals. These effects are especially important in view of the low hydrochloric acid output in young animals, as described before (Freitag, 2007).
Most of these data stem however from monogastric livestock, such as pig. Its investigation in aquaculture diets has been done only very recently.
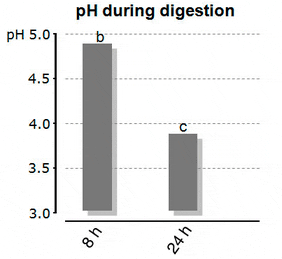
Bucking and Wood (2009) looked into the effect of feeding onto the stomach pH. The authors fed rainbow trout (mean weight 350 g) a commercial trout feed with 41% crude protein in a single-meal (2% body weight ration) and monitored the resulting pH in the stomach. Just before feeding the stomach pH was at ?2.7, whereas the pH one hour after feeding went significantly up to pH 4.9. It remained there for at least 8 hours, thereby being far above the optimum for pepsin activity. The chyme was released into the duodenum 8 hours after feeding, at far too high pH-levels. The authors speculated that the buffering capacity of the feed was a major contributor to the increased pH of gastric fluids. It took the fish more than 24 hours to reach the “low” initial pH of the stomach (Figure 1a and 1b). The effect of diet buffering capacity on the gastric acidification in juvenile fish was proven as well by Marquez et al. (2011a). They found that fishmeal diets had a 10 time higher buffering capacity, and needed therefore more energy for acid secretion per digestion cycle, than test-diets without animal protein meals.
Figure 1a: Stomach pH in Trout Before and Just After Feeding
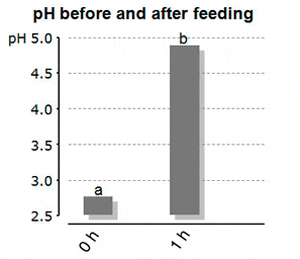
Figure 1b: Stomach pH in Trout During Digestion
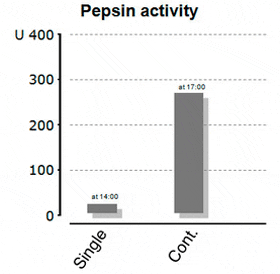
A more recent study from Yufera et al. (2012) took this a step further. This time, the authors not only looked for gastric pH, but evaluated the connection between stomach pH and pepsin activity in juvenile marine fish. Fish were either fed a single-meal (9:00), twice (9:00 and 17:00) or continuously (between 9:00 and 21:00). The gastric pH differed considerably among treatments. Fish which had been fed only once had pH-levels in the stomach around 4.5, while the highest pepsin activity was actually reported before (!) the feeding with 30 pepsin activity units per fish. Contrary to that, continuously fed fish reached a minimum pH in the stomach of ?2.5 and had a resulting pepsin activity of almost 280 units per fish in the late afternoon – clearly demonstrating the impact of low pH on pepsin activation (Figure 2a and 2b).
Figure 2a: Stomach pH in Differently fed Marine Fish
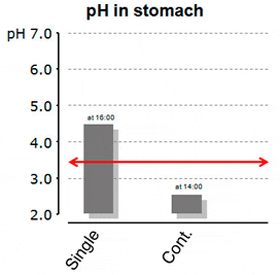
Figure 2b: Resulting Pepsin Activity in the Stomach of Marine Fish
What are the Implications?
The use of acidifiers is gaining more interest in recent years (Lückstädt, 2008). However, most of the described effects were believed to come from the anti-bacterial mode of action of organic acids. The impact on protein digestion is often overlooked. A recent meta-analysis for potassium diformate (Aquaform, ADDCON) found significantly improved weight gain and feed efficiency for tilapia in levels which can be already described as “growth promotion” (Lückstädt, 2012). These results may not come only from the surely existing anti-bacterial effects. Since acidifier, if chosen properly, have impacts on buffer capacity and/or stomach pH they will also have an impact on the digestion processes in the gastric tract.
July 2013


