In commercial production of trout and other salmonids in the United States, eggs are typically produced
on broodfish farms that are separate from farms used for the production of fish for food or for stocking. The production of good quality, disease-free eggs is a specialized activity requiring a high
degree of skill and management. Most of the eggs used in commercial trout production in the southeastern U.S. are produced in the Pacific Northwest. Trout eggs are usually shipped when they reach
the eyed stage (Fig. 1), which is more than halfway through the incubation period.
Incubation time is temperature dependent. At 55 °F, rainbow trout eggs will hatch approximately
3 weeks after fertilization, or within 4 to 7 days after being received as eyed eggs. At 45 °F,
the eggs will hatch approximately 7 weeks after fertilization.
Receiving and disinfecting eggs
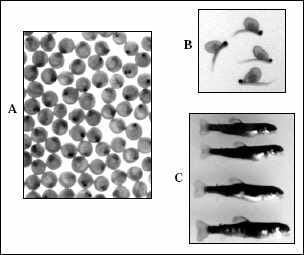 Figure 1. Eyed eggs (A), sac fry (B), and small fingerlings (C) of rainbow trout (90 percent lifesize). |
Gently add the eggs to the water, then add small amounts of clean water from your hatchery to gradually raise the temperature of the eggs. The eggs need to be gently stirred once or twice during the tempering process to ensure adequate water circulation to all eggs. Tempering should not be done in your hatchery, but should be done in a separate area to avoid possible contamination of the hatchery with disease organisms. Also, discard or destroy all shipping containers to prevent contamination of the hatchery.
The eggs should have been disinfected by the supplier before shipping, but it is good practice to also disinfect them yourself. Do not disinfect eggs that are already hatching. Disinfectants used for this procedure contain iodine (polyvinylpyrrolidone iodine), and should be administered as a 10-minute bath treatment at 100 parts per million (ppm) of free iodine. Typical iodophor disinfectants such as Argentyne® or Betadine® contain 1.0 percent (10,000 ppm) available iodine. A 100 ppm iodine concentration is equivalent to a 1:100 dilution of jug strength solution (use 38 milliliters, or 1.28 fluid ounces, or 7.5 teaspoons per gallon of water).
Other iodophors such as Wescodyne ® contain 1.6 percent or greater available iodine and require more dilution (use only 23.7 ml, 0.8 fluid ounces, or 4.5 teaspoons per gallon of water). Be sure of the concentration of the compound you are using. In poorly buffered (alkalinity < 35 ppm) or acidic waters, you should add sodium bicarbonate at 0.5 grams (approximately 0.5 level teaspoons) per gallon of water to buffer the water before disinfecting. Gently mix eggs and disinfectant to ensure that all egg surfaces are treated. Rinse the eggs with clean water to remove iodine residue before putting them into incubators. The eggs may now be counted and placed in incubators for hatching.
Egg enumeration
Many different methods can be used to count eyed trout eggs, including measuring volume, displacement or weight; counting the number of eggs in a V-trough (Von Bayer method); or using electronic egg counters. Most trout farmers use the displacement method to estimate egg volume because it is relatively fast, simple, accurate, and does not require much equipment. The numbers of eggs per unit of volume can be estimated by counting 50 eggs into 25 ml of water in a 50-ml graduated cylinder or burette, and noting the increase in volume. Repeat the process three to five times and calculate the average displacement. The number of eggs per milliliter or per fluid ounce can then be estimated using Table 1.
Egg incubation
Three types of incubator systems are commonly used: California trays; vertical tray or Heath incubators; and upwelling incubators (Fig. 2). California trays are screened, flat-bottomed trays
that fit inside rearing troughs in a horizontal series. Between each tray, a partition extending to the
bottom of the trough forces water through the eggs from below.
California trays are used primarily when very large numbers of eggs are being incubated and the water supply is not limited. The sac fry and egg shells are difficult to separate in these trays, and
eggs are often removed from these trays before hatching begins. California trays are rarely used in
the South.
Vertical tray incubators are essentially California trays arranged in stacks. They require relatively little floor space or water to incubate large numbers of eggs. The water is re-aerated as it flows
through the stack of trays. The recommended water flow in vertical tray incubators is 4 to 6 gallons
per minute (gpm). Vertical tray incubators require that the egg shells be manually removed after hatching, but the sac fry can be kept in the trays until swim-up at about 10 to 14 days after hatching.
To prevent smothering, trout eggs should be placed no more than two to three layers deep in vertical
incubator trays.
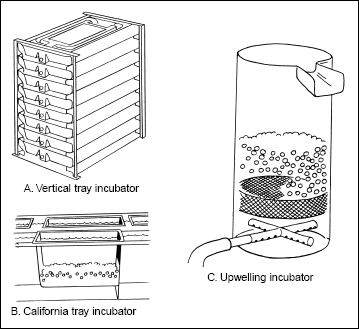 Figure 2: Incubators used in trout production. Vertical tray and upwelling incubators are most frequently used. |
All types of egg incubators should be covered to protect developing embryos from direct light. To accomplish this when using vertical trays, do not put eggs in the top tray. Eyed trout eggs purchased from a commercial supplier will usually contain a very small percentage of dead eggs, and may not require treatment for fungus. However, if the eggs are more than 3 days from hatching, dead eggs should be removed regularly to limit fungal infections. Removing dead eggs is more effective than chemical treatment at controlling fungus, but can be very time consuming.
If it becomes necessary to control fungus chemically, add formalin to the inflowing water to achieve a dilution of 1:600 (1,667 ppm) for 15 minutes daily. This is equivalent to 95 milliliters of formalin per gpm of water flow, or 3.2 fluid ounces of formalin per gpm of flow added over a 15-minute period. Trout eggs should not be treated with formalin within 24 hours of hatching, as the eggs will die. Hydrogen peroxide also can be used to control fungus on trout eggs, but is not widely used by hatchery managers in the South. Once hatching begins, the eggs and sac fry should not be treated with any chemicals.
Handling sac fry
Hatching rate depends on water temperature, but usually will be completed within 2 to 4 days after
commencing. Empty shells should not be allowed to accumulate in the incubating units. If the eggs
are incubated separately from the rearing troughs, the sac fry should be transferred into troughs shortly after hatching is complete. Up to 30,000 fry can be stocked into a standard fry trough 10 feet long and 18 inches wide. The water flow rate should be 8 to 10 gallons per minute for most facilities.
Keep the water level in the trough fairly shallow (3 to 4 inches) and the flow reduced until fry swim
up, approximately 2 weeks after hatching at 55 oF. Any dead fry, egg shells or deformed fish should be removed regularly.
When about 50 percent of fry swim up, begin feeding with a small amount of starter mash on the surface three to four times daily. Continue until most fish are actively feeding. Feed every 15 minutes if possible, but not less than hourly at this stage. A large kitchen strainer makes an excellent
tool for distributing the starter feeds throughout the tank. Automatic feeders are certainly more convenient than feeding by hand, but many are not well suited to distributing the smallest feed sizes.
Feed approximately 10 percent of the fish weight per day for 2 to 3 weeks, or until fry are about 1 inch long (approximately 1,000 per pound); then feed according to a published feeding chart. Fry feed should be formulated to contain approximately 50 percent protein and 12 to 15 percent fat. Excess feed and fish waste must be removed from the troughs at least daily. Small paintbrushes or feathers work well for cleaning the rearing troughs.
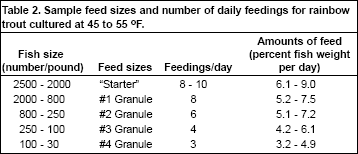
Siphons also can be used if care is taken to avoid the fish. After the fry have been actively feeding for 2 weeks, sample count the fish every week and adjust the feeding rate and feed size accordingly
(Table 2). Adjust the fish densities in the troughs as necessary to prevent overcrowding. In the standard fry tank described previously, fish are typically kept below 1 pound of fish per cubic
foot of water volume. Monitoring dissolved oxygen levels will help determine when fry density should be reduced. Ideally, the dissolved oxygen level should not be lower than 6 ppm. The fry will be ready to move into larger tanks in the hatchery when they grow to 1 inch in length. In areas where Yersinia ruckeri, the causative agent of enteric redmouth disease (ERM), has been detected, the fish should be vaccinated 2 weeks before moving them to a production facility. The recommended minimum size for immersion vaccination of trout against ERM is 4.5 grams, or approximately 100 fish per pound.
Hatchery sanitation
Restrict personnel traffic to reduce the possibility of contaminating the hatchery with disease. The use of a disinfectant footbath just inside hatchery doorways is a good preventative measure. All equipment used in the hatchery should be reserved exclusively for hatchery use. Clean and disinfect the hatchery and equipment regularly with a hypochlorite solution, or an approved quaternary ammonium disinfectant. Troughs and floors also should be disinfected between groups of fish. As an additional safeguard against the spread of disease microorganisms, keep the hatchery well ventilated to prevent condensation from forming on walls or on the ceiling.
Further Information
For more information on Trout Production, see the links below:
- Budgets for Trout Production: Estimated Costs and Returns for Trout
- Trout Farming: Carrying Capacity and Inventory Management
- Trout Production: Feeds and Feeding Methods
- Rainbow Trout


