The breeding and seed production of high value marine fin fishes has been expanding in recent years internationally.
Large quantities of hatchery produced seeds meet the need for sea cage farming in many countries (Hong and Zhang, 2003). It is well understood that the first step towards seed production technology is the development of broodstock. Prior to the 1980s, broodstock of fin fishes were grown mainly in indoor concrete tanks. Since then, wild-caught broodstock have been developed either in outdoor earthen ponds or in sea cages. It has been proved that broodstock development in sea cages was highly effective in improving gonadal development for most species like cobia, groupers, pompano, red seabream, Japanese flounder and yellow croaker.
The development of hatchery technology for commercial level seed production of marine fin fishes is still in its infancy in India, except for the seabass, Lates calcarifer. Hence, research and development need to be focused in evolving technologies for the seed production and farming of high value marine food fishes.
In recent years the seed production and farming of cobia (Rachycentron canadum) has been rapidly gaining momentum in many Asian countries (Liao and Leano, 2007). Cobia is distributed worldwide in warm marine waters. They are found throughout the water column and are caught in both coastal and continental shelf waters, although they are typically considered to be an offshore species. Wild-caught cobia does not support a major commercial fishery and generally considered as incidental catch. Sexual maturity is reported in males at one to two years and in females two to three years, with females growing both larger and faster with maximum sizes upto 60 kg. (Shaffer and Nakamura, 1989).


Fast growth rate, adaptability for captive breeding, lowest cost of production, good meat quality and high market demand especially for sashimi industry are some of the attributes that makes cobia an excellent species for aquaculture. Under culture conditions, cobia can reach three to four kg in body weight in one year and eight to 10 kg in two years.
The species has protracted spawning season and it can spawn in captivity. The fecundity is very high. Aquaculture research with cobia was first reported in 1975 with the collection of wild caught cobia eggs off the coast of North Carolina. Larval development was described and after the termination of 131 day rearing trial it was concluded that cobia had good aquaculture potential because of its rapid growth and good flesh quality. Additional research on cobia was conducted in the late 1980s and early 1990s in the USA and Taiwan, Province of China.
Research continued and by 1997 the technology to raise large quantities of cobia fry had been developed and Taiwan Province of China was producing cobia juveniles for grow out mostly in nearshore cage systems (Yeh, 2000; Su et al., 2000; Liao et al., 2004; Liao and Leano, 2005). Cobia production is also reported in the United States, Puerto Rico, Bahamas, Martinique, Belize, Brazil and Panama (Bennetti et al., 2008). Envisaging the prospects of cobia farming in India, broodstock development was initiated at the Mandapam Regional Centre of Central Marine Fisheries Research Institute in sea cages during 2008 and the first successful induced breeding and seed production was achieved in March – April 2010.
Broodstock development and captive breeding at Mandapam
The broodstock at Mandapam were developed in sea cages of six meter diameter and 3.5 m depth (Gopakumar, 2008). Wild collected cobia brood fish in the size ranging from two to 10 kgs of weight were stocked during December 2008 to February 2009. The fish were stocked without separating sexes. All the fish were collected by commercial hook and line fishers.
After transporting to hatchery the fi sh were treated with 100 ppm formalin for two to five minutes and conditioned for two to three days in 10 tonne FRP tanks before transferring to cages. These fishes were fed twice daily at 0900 and 1530 hrs with sardines and other species such as Pellona and Ilisha , and occasionally with squids and portunid crabs at five per cent of their body weight.
Vitamin and mineral supplements were also given twice in a week along with feed in order to complement any possible nutritional deficiencies in their diet. A total of 40 fishes were stocked in four cages. The length range and corresponding weight range of the brood fishes recorded during April 2009 ranged from 80-127 cm and four to 20 kg, respectively. The sexes were separated by cannulation using a flexible catheter (two mm inner diameter) in June 2009 and stocked in separate cages. Thereafter the females were cannulated every fortnight to assess the diameter of the intra-ovarian eggs.
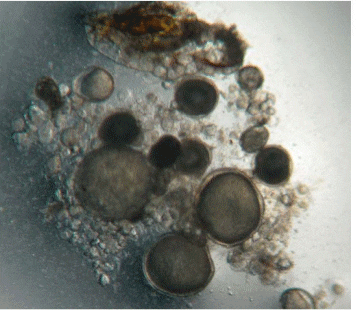
In March 2010, one of the female with intra-ovarian eggs of around 700 μ size, was selected for induced breeding. The size of the female was 120 cm in total length and 23 kg in weight. Two males were also selected from the male cage. The males were 100 cm and 103 cm in total length and weighed 11 kg and 13.5 kg, respectively. The selected brooders were introduced in a 100 tonne roofed cement tank with about 60 tonnes of sea water on the same day. At around 1300 hours, the brooders were induced for spawning with HCG at doses of 500 IU per kg body weight for female and 250 IU per kg body weight for males. Spawning was noted at 0430 hours on 13.03.2010. The total eggs spawned were estimated as 2.1 million. About 90 per cent fertilisation was recorded (fertilised eggs amounted to 1.9 million). The eggs were collected by a 500 μ mesh and stocked in incubation tanks with varying densities.

The eggs hatched after 22 hours of incubation at a temperature range of 28-30º C. The percentage hatching was 80 per cent and the total number of newly hatched larvae was estimated at 1.5 million. The newly hatched larvae measured in total length from 2.2-2.7 mm. The mouth opening was formed on the third day post hatch and was measured at around 200 μ. The environmental conditions during the larviculture period were DO2: > 5mg / l , NH3: < 0.1mg / l, pH: 7.8 – 8.4, Salinity: 25-35 ppt, water temperature : 24-33° C (Liao et al., 2004).
Larviculture protocols followed at Mandapam
Larviculture protocols were developed by appropriate management of live feeds in suitable quantities and also taking into consideration the nutritional requirements of the larvae. The larvae were stocked in FRP tanks of 5 ton capacity for larviculture. The intensive larviculture tanks were provided with green water at a density of about 1x105 cells per ml and rotifers enriched with DHA SELCO at a density of six to eight per ml from three to nine days post hatching.
The critical stage for the larvae was days five to 7seven post hatching when they entirely resorted to exogenous feeding from yolk sac feeding. During this period, large scale mortality (about 80 per cent) was noted. Thereafter, the mortality rate was moderate. From days nine to 21 post hatching the larvae were fed four times daily with enriched Artemia nauplii by maintaining a nauplii concentration of two to three individuals per ml.
During this period, co-feeding with rotifers was also continued due to the presence of different size groups of larvae. Green water was also maintained in appropriate densities in the larval tanks. From 18 days post hatching onwards, the larvae were fed with newly hatched Artemia nauplii and weaning to larval inert feeds was also started as per the details given in the table.
From 25 days post hatching, grading of larvae was started. The shooters were fed exclusively with the artificial feed of the size 500-800 μ and 800-1200 μ. At 30 days post hatching, three size groups of juveniles were noted with mean sizes of 10 cm (10 per cent), six cm (25 per cent) and four cm (65 per cent). The juveniles measuring 10 cm length were ready for stocking in hapas and the other two size groups would be ready after rearing for another two to three weeks. All the fingerlings of 10 cm length and above were stocked in hapas in the sea for nursery rearing for about a month before transferring them to the grow out cages.
Table 1. Age and feed size in weaning of cobia fingerlings
| Stage of larvae (days post hatch) | Size of larvae (cm) | Size of feed (μ) |
|---|---|---|
| 18 – 19 | 2.3 – 2.6 | 100-200 |
| 20 – 23 | 2.5 – 3.5 | 300-500 |
| 23 – 30 | 3.5 – 8.0 | 500-800 |
| 31 onwards | > 4.0 | 800-1200 |
October 2011


