What makes
dissolved oxygen concentration so
important in intensive fish culture
is the speed with which it can
change. Over a matter of hours, or
sometimes even minutes, DO can
change from optimum to lethal levels.
No other critical environmental
variable in fish culture is so
dynamic.
The dynamic nature of dissolved
oxygen results from the interaction
of three factors. First, oxygen is not
very soluble in water so water has
only a limited capacity to hold
oxygen. Second, the rate of oxygen
use by fish, plankton and organisms
living in the pond mud can be
high. Third, oxygen diffuses very
slowly from the atmosphere into
undisturbed water. The combination
of these three factorslimited
solubility, rapid use and slow
replenishmentcan cause rapid
changes in dissolved oxygen concentrations.
Dissolved oxygen levels can be
managed with aeration, but the
response time for taking corrective
measures is short. This makes it
critical to have a rapid and reliable
method of measuring dissolved
oxygen concentrations so that aeration
devices can be activated when
needed.
There are a number of ways to
measure dissolved oxygen concentration.
Select a method based on
1) the number of ponds or tanks to
be measured, 2) the level of accuracy
required, and 3) the cost of the
measurement technique.
The titration-based drop count
method fairly rapidly assesses
whether or not there is sufficient
dissolved oxygen in water. The
drop count method is inexpensive
and appropriate if DO concentration
is to be measured infrequently
in a few ponds or tanks. However,
on commercial fish farms or in any
other situation where DO measurement
of many ponds or culture
units is routine, a dissolved oxygen
meter is an indispensable piece of
equipment.
What is an oxygen meter?
An oxygen meter has two components
the sensor (sometimes
called the probe) and the meter.
Various types of sensors are available,
but they all operate in basically
the same way: the sensor
reacts with oxygen and an electrical
signal is produced in proportion
to the oxygen concentration.
The signal is then amplified, translated
into concentration units, and
displayed by the meter. The meter
circuitry also compensates the
reading for changes in temperature,
altitude or salinity. The
meter circuitry may also include
features to aid in calibration.
Most DO sensors operate as electrochemical
cells with a positive
electrode (cathode) and a negative
electrode (anode) connected by a
salt bridge consisting of a saturated
electrolyte solution. In most
sensors, oxygen passes through a
permeable membrane and is
chemically reduced within the
sensor. The chemical reduction of
oxygen generates an electrical current
that is processed by the electronic
components within the
meter and displayed as a DO concentration.
The current is proportional
to the concentration. Thus,
DO meters do not measure oxygen
concentration directly, but measure
a voltage that is produced by
the chemical reactions of oxygen
with the various components of
the sensor.
Types of dissolved oxygen sensors
Polarographic or Clark sensors
use gold or platinum as the cathode
and silver as the anode (Fig.
1). Polarizing voltage is applied to
the cathode to cause the reduction
of oxygen within the sensor.
Oxygen is consumed at the cathode
according to the reaction: O2 +
2H2O + 4e- 4OH-. In response to
the production of hydroxyl ions
(OH-) at the anode, and in order to
preserve the charge balance of the
electrolyte (saturated KCl) solution,
chloride ions react with silver
at the anode according to the reaction:
Ago + Cl- AgCl. Therefore,
the chloride ions in the electrolyte
solution function as a carrier of
the electric potential.
Galvanic sensors use silver or
platinum as the cathode and lead,
iron or zinc as the anode.
Application of a polarizing voltage
is not necessary because the reduction
of oxygen in the presence of
the sensor materials is spontaneous.
Thus, a galvanic sensor is
like a battery (fuel cell) that is
fueled by oxygen. Galvanic sensors
typically have faster response
times than polarographic sensors
and are more expensive.
Fiber optic oxygen sensors consist
of an optical fiber with a sensor tip
that contains a thin layer of oxygen-
sensitive fluorescent dye dissolved
in pure silicon. The optical
fiber carries blue light from a lightemitting
diode (LED) to the sensor.
This stimulates the dye to emit fluorescent
light that travels back up
the optical fiber to a photodetector.
Oxygen diffusing into the sensor
tip binds to the fluorescent dye,
which reduces (quenches) the
intensity of light emission. The
extent of quenching is directly
related to oxygen concentration.
Fiber optic sensors are very sensitive
at low DO concentrations.
Fiber optic sensors are sensitive to
ambient light, but this problem can
be overcome by coating the sensor
tip with silicon. However, this silicon
overcoat will reduce probe
response time.
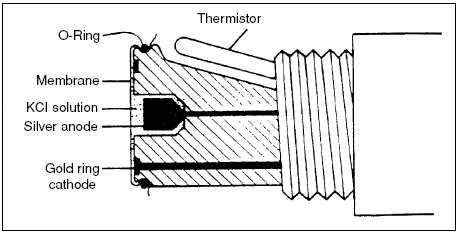
Figure 1. A cross section of a typical polarographic dissolved oxygen sensor.
Which oxygen meter is best?
Many different oxygen meters are
commercially available (see list of
manufacturers below), and each
model has a unique combination of
features that makes it more or less
suitable for a particular application.
The best meter for occasional
use in an indoor setting, such as a
hatchery, will be quite different
from the one that is best for regular
use under rough, outdoor conditions.
The purchase decision is further
complicated by the fact that
good meter systems are expensive,
primarily because precious metals
are used in the construction of
many sensors. Before buying a
meter, consult with other fish farmers,
Extension specialists, aquaculture
supply companies, and meter
manufacturers to identify the most
suitable one.
Some of the desirable features of a
dissolved oxygen meter suitable
for making field measurements
include:
- accuracy
- rapid response
- ease of calibration
- water resistance
- sturdy, rugged construction
- automatic temperature compensation
- manual salinity compensation
- manual barometric pressure compensation
- measures from 0 to 200 percent saturation
- easily changed cable or probe
- at least a 25-foot cable
- a digital, liquid crystal display that can be read in bright sunlight or in total darkness
- an integral membrane cap assembly
- a built-in calibration chamber/storage sleeve
- storage of measured values in memory within the meter (datalogging)
- an RS-232 personal computer interface
- a hold or auto-read function indicating that a stable reading has been attained
- a battery charger
Operating an oxygen meter
It is beyond the scope of this publication to provide detailed operational instructions for each of the many kinds of meters. Carefully read the instructions that come with the meter to understand how it works and how to use it properly. Remember, the number displayed by the meter is not necessarily accurate. The number on the display is correct only when the meter has been properly calibrated, the measurement is made correctly, and the sensor and meter have been properly maintained. One common step in the use of most meters is calibration, and some details of this process are presented in the next section.
Further Information
To continue reading this article, please click here (PDF)
Source: Southern Regional Agricultural Center and the Texas Aquaculture Extension Service - Taken from site - January 2006

