Globally, tilapia aquaculture has been expanding rapidly to now represent the third most important finfish aquaculture species group after the Chinese and Indian Major Carps (SOFIA 2012). Much of this growth has been fueled by the availability of fast growing strains of the Nile tilapia (Oreochromis niloticus), improved through selective breeding to produce fish that can uniformly reach market sizes of 800 g in 10 months or less. However, much concern has been expressed by the conservation community that widespread introduction into Africa of selectively bred O. niloticus could result in the destruction of conspecific and other indigenous tilapia biodiversity that, given appropriate environmental impact regulation and breeding programs, could also be used productively and profitably in aquaculture.
Quality of Farmed Stocks
Tilapias exhibit a high degree of phenotypic plasticity. When grown in small ponds, they often mature precociously and these small adults are mistakenly stocked as fingerlings.
Instead of growing, they reproduce, which in practice effectively amounts to inadvertent selection for slow growth and/or early sexual maturation (Doyle 1983) and can lead to declines of up to 20 percent in growth rate within six generations (<three years) (Silliman 1975).
Since it is expensive, difficult, and sometimes illegal to get good broodfish, individuals who seek to acquire and maintain a hatchery population, often resort to using minimal numbers, thus building low genetic diversity into their production systems from the outset (Eknath 1991). Often the effective number of broodfish used to contribute to each subsequent generation (Ne) is less than 100-150 pairs needed to maintain healthy genetic variability and minimize in-breeding (Smitherman & Tave 1987). Even when numbers are sufficient, simply stocking 100 males and 100 females into a brood pond will not solve the problem. As male tilapia are highly territorial and competitive for mates, only 30% of males dominate the fertilization of the females (Fessehaye et al. 2006). Rates of inbreeding in such systems are about double that expected in a randomly breeding population (Fessehaye et al. 2006).
Many Nile tilapia introductions were of only a small number of individuals or of limited family representation (Pullin 1988; Agustin 1999). The Bouaké research station in Côte d’Ivoire is characteristic of how Nile tilapia genetic diversity has been managed. Captured in Burkina Faso in the 1950s and transferred to Bouaké, what was originally a pure population of O. niloticus was hybridized with other strains and species and subsequently widely distributed in Africa and elsewhere (Thys van den Audenaerde 1988). Transfers from Bouaké to Paraguay (1968), Sierra Leone (1970), Venezuela (1971), Brazil (1971), Bénin (1979), Guinea (1978 and 1983), Mali (1982), and back to Burkina Faso (1982) have been reported (Adou, in R.S.V. Pullin, 1988). The Bouaké case is by no means unique, with many such introductions and crosses having been made over the years, either purposefully or by accidental mixing. For example, the Baobab Fish Farm Kenya, until recently (they are now no longer operating) grew a hybrid strain of O. niloticus, Oreochromis spilurus, and O. mossambicus. Kafue Fish Farm in Zambia maintains stocks of O. niloticus, Oreochromis andersonii, Oreochromis aureus, and gets wild Oreochromis mortermeri, and Oreochromis macrochir from the Kafue River. All of these species easily cross with each other and perform less well than the original O. andersonii stock (F. Flynn pers comm, Lusaka, Zambia, Sept 2003). In general, the worst problem with such composite populations from the fish farmer’s point of view is that they exhibit very high variability in performance among individuals and generations.
Another negative consequence of this mixing is outbreeding depression, through which the fitness of populations that have adapted over time to culture conditions decreases through dilution with genes from other populations or species. Today, most research stations and private tilapia hatcheries around the world maintain and disseminate strains comprised of mixtures of several species derived from a number of sources.
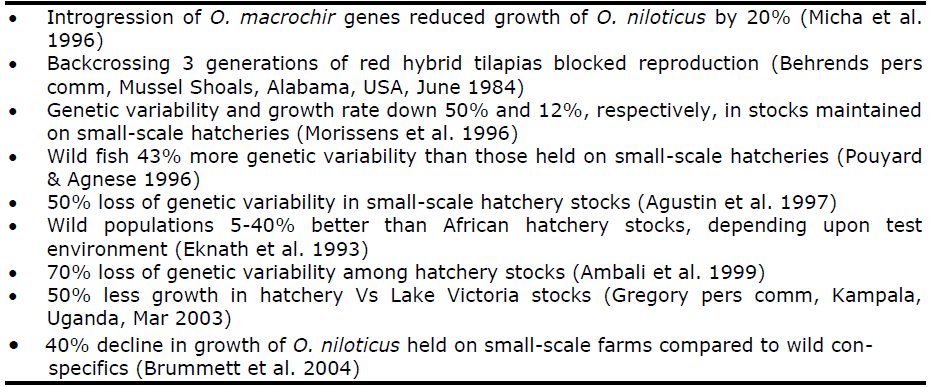
Substantial declines in performance are associated with such hatchery management practices. Genetic variability is the way in which selective breeding improves growth. Typically, genetic variability of fish held in African hatcheries is 40-70% less and growth rates 12-40% less than wild stocks (Table 1).
Response to selective breeding can be reduced by up to one third by even moderate reduction of genetic variation (Bentsen & Olesen 2002). Heritability, the percentage of total variability that can be inherited, is of utmost importance to breeders. Heritability (h2) for growth in tilapias is naturally low to moderate, averaging less than 20 percent and can easily be swamped by low Ne (ICLARM/UNDP 1998). Even with Africa’s relatively small aquaculture industry, a 20 percent decline in growth rate represents lost production on the order of 80,000 t, with associated loss of profits on the order of $200 million per year to Africa’s fish farmers (FAO 2000)
Genetic Management
The first successful tilapia genetic improvement program was carried out by the WorldFish Center (the International Center for Living Aquatic Resources Management) and Central Luzon State University in the Philippines. The program was based on a combined family selection program that resulted in increased growth rate of 64% over eight generations, in a composite strain comprised of eight different wild and farmed populations (Khaw et al. 2008). This genetically improved farmed tilapia (GIFT) has been widely disseminated in Asia where it substantially out-performs most local strains (Asian Development Bank 2005). Although illegal introductions are suspected, GIFT has not been legally re-introduced to Africa for commercial farming. An on-station comparison of the GIFT and Akosombo strains of O. niloticus that could lead to the first major introduction, is underway in Ghana. Since the development of GIFT, a number of improved strains, some based on GIFT, such as Big Nin from Thailand, and GET-Excel and FaST, from the Philippines have been produced.
Growth comparisons have shown significant differences among both wild and captive populations and relatively low genotype x environment (G X E) interaction in the environments tested, indicating that lines improved under one set of culture conditions will continue to perform well in other systems (Khater and Smitherman 1988; Eknath et al. 1993; Asian Development Bank 2005).
The territorial nature of O. niloticus lends to rapid formation of distinct sub-populations, a characteristic that if reinforced by long-term geographical separation and genetic drift has led to a certain amount of genetic divergence (Nyingi 2007). There are seven subspecies of Nile tilapia in their natural ranges: O. niloticus niloticus, the largest group representing populations in West Africa and the Nile River valley; O. n. eduardianus in Lakes Edward, Kivu, Albert, and Tanganyika; O. n. cancelatus in Ethiopia; O. n. barengoensis in Lake Baringo; O. n. vulcani in Lake Turkana; O. n. sugutae in the Sugutu River of Kenya; and O. n. filoa in a hot spring in the Awash River basin of Ethiopia (E. Trewavas, 1983). An eighth subspecies from Lake Tana has been identified (S. Seyoum and I. Kornfield, 1992). This is based on micro-satellite and mitochondrial DNA analyses. A ninth subspecies has been found in a warm water spring in the Loboi Swamp near Lake Bogoria in Kenya (D. Nyingi, 2007). Overall, heterozygosity (less than 4.5 percent) and gene polymorphism (less than 3%) is relatively low in Nile tilapia compared to some other fish species (Gourène and Agnèse 1995). As hatchery managers have learned, Nile tilapia easily hybridize with other oreochromiines both in the wild and in captivity, although some of these crosses produce sterile offspring or skewed sex ratios (Wohlfarth and Hulata 1983; Agnèse et al. 1998).
Proper management of captive tilapia genetic resources is somewhat complicated but not impossible. In those few cases where the genetic diversity of hatchery stocks has been systematically managed either through the use of large effective breeding numbers, rotational mating or controlled outcrossing, growth rates equal or exceed those of natural populations (Pauly et al. 1988). Selective breeding can lead to increases in growth rate of 10-15 percent per generation (Jarimopas 1990, Rognon & Guyomard 1996, Vreven et al. 1998, Ponzoni et al. 2011).
Improvements in genetic quality undoubtedly increase production. In order to be profitable, crop agriculture relies heavily on improved varieties. However, how these improvements are made can have a large impact on the rate of progress, who benefits, and how. There are two general approaches to improving the genetic quality of fish raised in aquaculture: 1) import an exotic species or improved variety developed elsewhere (centralized approach) or 2) locally develop a new species or variety (decentralized approach).
Agricultural research and development have long relied on the option of domesticating or breeding a new variety in a central location and subsequently disseminating seed or broodstock. This approach requires the international and often intercontinental transfer of genetic material thus risking both negative environmental impacts and the possibility of G X E interaction, which renders improved genotypes less competitive in culture systems that differ from those under which they were bred.
The major advantage of the centralized approach is that complicated technologies can be more easily managed in larger, more sophisticated facilities. Breeding progress is faster. For example, the GIFT strain grows 20-70 percent faster than most captive O. niloticus strains (ADB 2005). However, in Thailand and China, the GIFT exhibits signs of G X E interaction, which renders improved genotypes less competitive in culture systems that differ from those under which they were bred.
The major advantage of the centralized approach is that complicated technologies can be more easily managed in larger, more sophisticated facilities. Breeding progress is faster. For example, the GIFT strain grows 20-70 percent faster than most captive O. niloticus strains (ADB 2005). However, in Thailand and China, the GIFT exhibits signs of G X E interaction and is not substantially superior to locally adapted and bred strains under certain conditions. The GIFT was produced in four years while the strains in Thailand and China were slowly selected over 30 and 20 years, respectively (ICLARM 1998).
Decentralized genetic improvement to produce specific lines of fish for specific culture systems normally takes longer than the centralized approach. Decentralization requires more people to be involved and is consequently less efficient in terms of capital use. It also tends to be more difficult to implement in short-term projects preferred by government. On the other hand, one of the key constraints to improved genetic management of African aquaculture species is the lack of skilled technicians and hatchery infrastructure. Without the capacity to undertake proper management and breeding, the potential gains inherent in a new strain will be quickly lost. The on-the-job training opportunities created by decentralized genetic improvement projects are excellent means of creating both new strains for culture and the capacity to manage them at the same time. Unfortunately, progress to date on building this capacity in national hatcheries in Egypt, Malawi and Ghana has been less than stellar partly due to poor funding, but also and importantly, due to lack of commitment.
Economic Importance of Genetic Improvement
Tilapia are a global commodity, with 350,000 metric tons traded internationally for over US$800 million each year (FAO 2013). The market is dominated by large-scale producers, mostly in China, who are able to put frozen tilapia onto markets throughout Africa at prices below local production costs, largely due to the use of faster-growing strains (M. Amechi, pers. comm., Accra, Ghana, Feb 2013; P. Blow & C. Chiwenda, pers. comm., Kariba, Zimbabwe, July 2013).
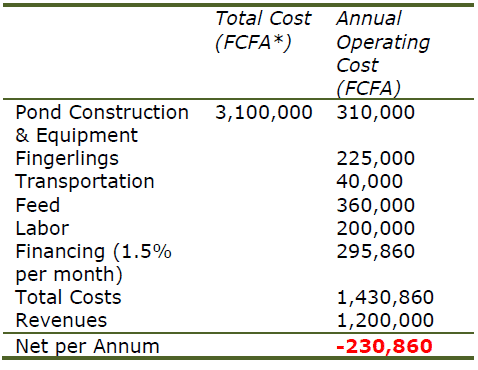
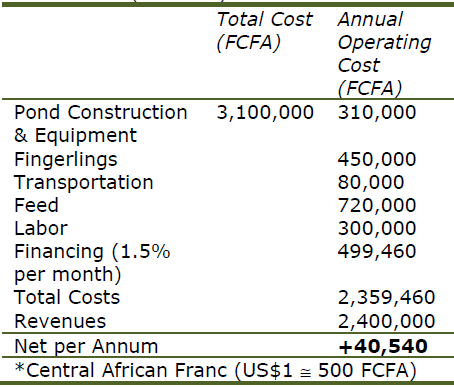
Competing with cheap imports of tilapia strains that grow nearly twice as fast as local strains is impossible, especially for smaller-scale farms. Simple enterprise budgets developed for tilapia farms in Cameroon illustrates the importance of genetics in aquaculture profitability (Table 2a,b).
The use of improved strains is essential for the success of African tilapia farming at the farm level and, extrapolated to the existing African tilapia industry, could easily double production to 1.5 million metric tons worth an additional US$2.2 billion per year.
Risks of Using Improved Lines in Africa
Theoretically, the use of improved strains could represent a threat to indigenous African tilapia populations in terms of declining abundance or reduction of genes with potential importance for future selective breeding programs. At present, there is insufficient data available on tilapia ecology and/or genetic diversity to permit fully informed decision-making in regard to the potential negative impacts on wild African tilapia stocks of introducing or developing new improved strains. However, the debate over the use of genetically modified fishes, either naturally through selective breeding and hybridization (among other techniques), or through transgenetic methods, has become global, and includes a large number of case studies particularly from Europe and North America. Whether or not these data are sufficient to adequately assess the risks involved in the use of selected tilapia strains for aquaculture is debatable.
Genetic Introgression
Cultured populations of indigenous species will inevitably escape and breed with wild fish. Declines in wild salmon runs have been attributed to the accidental escape of cultured salmon and/or the purposeful introduction of hatchery stocks (Fleming et al., 2000; Utter and Epifanio, 2002; McGinnity et al., 2003). Although impossible to differentiate from the combined effects of landscape modification, pollution and over-fishing, the process of genetic introgression, that is, the migration of genes from the captive population into the wild population through inter-breeding has been given as the principle mechanism behind these declines (Araki et al 2007). The overall degree of adaptation or fitness of the wild population could be reduced by mixing the genomes of captive fish that are specifically adapted to a hatchery or aquaculture environment, with those that are specifically adapted to a particular river, or by increasing the relative percentage of genes from one subset of the wild populations. (Ryman, 1991). The magnitude of this potential problem is proportional to:
1. The degree and importance of the adaptation of the wild population to the waterbody in question. In cases where only a narrow range of genotypes can survive in a particular waterbody, the genetic variability of the fish population narrows, rendering the wild population vulnerable to environmental changes, one of which is the presence of large numbers of fish of other genotypes.
2. The relative sizes of the captive and wild populations. In cases where relatively small (<1,000 individuals) wild fish populations that are highly adapted to a particular river or lake, are inundated by tens of thousands of stocked or escaped fish, such as is the case for many wild runs of Atlantic salmon (Salmo salar). The consequences of the reduction in fitness could be catastrophic and ultimately result in the extinction of the wild genome, even in cases where the total number of fish in the waterbody has actually increased (McGinnity et al., 2003).
3. The degree of difference between the genomes of the wild and captive populations. Up to the point where they can no longer interbreed at all, the more distant the relationship between the introduced and wild genomes, the greater could be the reduction in fitness. This works both ways, with fitness of both the wild fish and the captive populations (i.e., outbreeding depression).
4. The goals of having captive and wild populations in the same stream. If preservation of the indigenous genome is considered of primary importance, the fact that there is often more fish in a stocked waterbody (or one into which cultured fish have escaped) may be less of a priority than the relative fitness of the population.
In the case of small, highly adapted Atlantic salmon runs, with relatively narrow genetic diversity, the risk of introducing large numbers of less well adapted hatchery fish has been shown to reduce co-adapted gene combinations and whole lifetime population fitness, at least in the short term (McGinnity et al., 2003). The case is much less clear for species with relatively large and genetically diverse populations such as sea bass, cod, red drum and red sea bream that have also been heavily stocked or used in aquaculture (Youngson et al., 2001; Utter and Epifanio, 2002).
Incidences of ecological disruption attributed to the introduction of alien tilapias have been widely documented (Lever, 1996; Canonico et al., 2005; De Silva et al., 2006). Since fish introduced for aquaculture do escape to the wild, the introduction of any strain of tilapia to watersheds for culture or capture fisheries where it is not currently found, should be undertaken cautiously. It is recommended that countries survey and characterize their indigenous tilapia biodiversity prior to considering such an introduction.
The principal debate between wildlife conservationists and fish farmers regarding transfer of genetically improved tilapias between African watersheds revolves around the danger of genetic introgression with wild populations that probably contain genetic diversity of important adaptive significance. These may be lacking in captive populations and may be important in terms of adaptive capacity and/or the development of future aquaculture strains.
Although there is no compelling empirical evidence arguing either for or against the possibility of negative impacts resulting from the introgression of captive tilapia strains into indigenous wild populations, the substantial biological and ecological differences between tilapia and salmon (upon which most of the concerns over genetic erosion are based) imply that the risks of introducing improved tilapias for aquaculture might be significantly less than feared.
Referring to the list of decision making criteria listed above:
1. Tilapia are generalists, often exhibiting high degrees of phenotypic plasticity, but not normally genotypically adapted to specific water bodies.
2. Wild tilapia populations are generally huge, in excess of millions of individuals, while escapes from aquaculture are minimized, relative to intentional stocking programs, for example, by farmers trying to protect their investments.
3. In terms of growth performance, hatchery populations of tilapia can differ by 40-60% from wild populations, at least in terms of growth rate.
4. Food security and economic growth in impoverished communities are key concerns. In addition to the ethical issues involved, failure to address food security needs probably increases the threats to aquatic biodiversity posed by over-fishing and environmental degradation.
In my view, only number three (3) gives substantial cause for concern. If, for example, there are serious threats to a tilapia population of particular significance for local capture fisheries, or of special value as a locally adapted race, the large difference between captive and wild fish could represent a danger of eroding locally adapted gene sequences (Deines et al. 2014).
On the other hand, the dangers associated with these genetic differences are proportional to the absolute value of the difference, not whether the difference is positive or negative. In addition, existing tilapia hatchery populations in Africa have significantly diverged from wild populations, mostly negatively in terms of growth performance. In consequence, the risk of doing nothing may be similar to the risk of using centrally selected improved lines, without enjoying the productivity and economic gains.
Other Tilapias
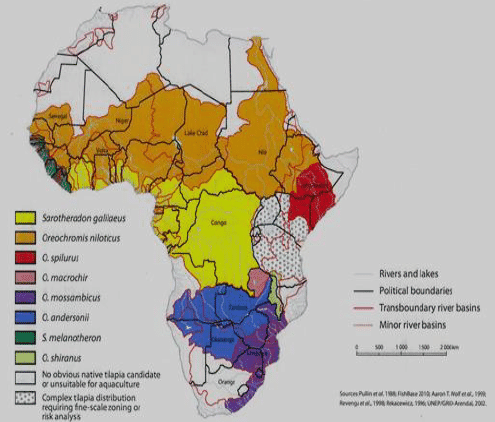
There are a number of species of tilapia in Africa, many of which have been tested in aquaculture. A few have demonstrated economic potential. These include: Oreochromis mossambicus and Oreochromis urolepis from the Lower Zambezi and environs; Oreochromis macrochir in the South-Central and S-Western parts of the continent; Sarotherodon galilaeus in the Center and West; Oreochromis andersonii in the upper Zambezi and Kafue Rivers; Oreochromis aureus in the Nilo-Sudan zone; Tilapia guineensis and Sarotherodon melanotheron in the coastal regions of West Africa; Tilapia rendalli in South-Central; and Tilapia zillii in Northern and Western Africa; and O. spilurus along the Eastern coast (Figure 2).
Many of these possess important culture traits for aquaculture: O. andersonii is a more placid and easily handled fish than O. niloticus; O. aureus is the most cold-tolerant of the tilapias and when crossed with O. niloticus produces all male hybrids that can be used in organic fish production; T. guineensis and S. melanotheron are tolerant of brackish water and the latter tends to mature at a later age than other species, potentially reducing the problems with precocious spawning (mentioned above). T. zillii and T. rendalli are herbivores that can help control weeds in ponds.
However, aquaculture is largely a start-up venture in most of Africa and faces many constraints. Having a fish like the GIFT strain of O. niloticus or one of several other similar lines, that grows 40-60% better than the typical farm populations can make the difference between success and failure. Improved lines of these other species are needed to encourage farmers to adopt local species and thus reduce pressure to further distribute O. niloticus. In addition, characteristics such as docility, salt tolerance, cold tolerance, and the ability to produce all male hybrids, deserve special concern and conservation effort.
Alternatives to Introduction
Some progress is being made in reducing incentives for importing alien lines and species. For O. niloticus, there are existing breeding programs at Akosombo in Ghana, and Abbassa in Egypt, that have reportedly produced 10-15% improvement in growth over several generations in spite of many problems (Joseph Ofori, pers. comm., Akosombo, Ghana, November 2013). For O. mossambicus, a breeding program has been established at Stellenbosch University in South Africa. O. shiranus, an indigenous species in Malawi, is being improved at the Malawi National Aquaculture Center, specifically to provide Malawian fish farmers with alternatives to O. niloticus, which is banned. There is also an on-going breeding program for O. aureus in Egypt. At least two fish farmers in Zambia are working to improve the performance of O. andersonii. However, it is imperative to do more, as pure populations of several species are under serious threat from genetic contamination. Among the most important species that remain, O. andersonii, O. macrochir, S. melanotheron and S. galilaeus probably have the highest potential to become economically viable aquaculture species.
Much emphasis has been placed on Best Aquaculture Practices and various certification schemes that seek to ensure environmental and social sustainability in the aquaculture sector. Consistent with these would be a zoning system to encourage fish farmers in various sub-regions to culture only indigenous species. A rough idea of what such a zonation might look like is shown in Figure 3. Any definitive zonation would have to take into consideration the many sympatric tilapia populations in Africa.
 The Aquatic Chicken
The Aquatic Chicken
Probably the only permanent solution that would protect the interests of farmers, consumers, and indigenous biodiversity, would be the creation of a truly domestic farm tilapia that possesses good culture traits while having low survivability in the wild. Aspects of this approach to fish genetic management were first proposed by Moav et al. (1978) and reiterated by Balon (2004), both working with common carp, Cyprinus carpio.
Tilapia has long been referred to as the “aquatic chicken” because of the presumed role it could play in aquaculture and human diets. However, a major difference between tilapia and chickens is that the former is essentially a wild animal (“exploited captive” according to the usage of Balon) and when escaping to the wild, can establish feral populations and interbreed with local tilapias. While chickens can, and do, interbreed with their wild progenitors, escaped chickens are generally fat, slow and dim-witted, and quickly done in by predators (Lawler 2012). Some strains of common carp and red tilapia exhibit traits that collectively would make them a true aquatic chicken:
- Easy seinability
- Sexual maturity above minimum market size
- Rapid growth
- Low aggression
- Good carcass quality (e.g., low fat)
- High fecundity
- Bright color (easy for predators to see)
- Slow-moving
- Specific disease resistance
- Grow under crowded conditions
- Convert low-quality feed
- Behavioral barriers to out-crossing
Some of these traits may be amenable to traditional selective breeding while others, such as disease resistance and feed conversion, might be more easily accomplished through direct manipulation of DNA. While currently anathema to many in the conservation community, the long-term environmental benefits of having a fully domesticated fish for tropical aquaculture would be a major contribution to global agriculture and food security, while significantly reducing the threat of introducing tilapias to new ecosystems.
July 2014