Amyloodinium ocellatum was described by Brown (1931) and is one of the most important pathogenic parasites affecting the culture of marine and brackish water fish (Noga and Levy, 2006). The parasite produces a powdery or velvety appearance on infected fish, and the resulting disease is commonly referred to as “marine velvet,” velvet disease, or amyloodiniosis. The organism is a dinoflagellate ectoparasite and has been reported in a wide range of marine and estuarine fish. It is one of a very few organisms that can infect both teleosts and elasmobranchs (Alvarez-Pellitero, 2008). This makes it a concern for public aquaria, which often have mixed species exhibits with representatives of each taxon.
This ectoparasite can be found on gills and skin (body and fins) of host fish. It can cause devastating disease and mortality because the organism is able to reproduce quickly when fish are crowded, especially in closed systems. This parasite has a broad host and geographic range, causing fish mortalities in tropical and temperate environments. Epizootics have been reported in feral and cultured fish, as well as in home and public aquaria. Rapid spread of the parasite and high mortality are common in cultured fish if the organism is not recognized and treated early in the course of an outbreak. A morphologically similar parasite, Piscinoodinium, infects freshwater fish but is much less common and less pathogenic than A. oscellatum.
While the infection of feral fish with A. oscellatum is not unusual, heavy mortality in wild populations is rare. Serious outbreaks have occurred in cultured red drum (Sciaenops ocellatus), striped bass (Morone saxatilis), striped mullet (Mugil cephalus), European sea bass (Dicentrarchus labrax and Lates calcarifer), pompano (Trachinotus sp.), yellowtail (Seriola dumerili), gilthead sea bream (Sparus aurata), and marine clownfish (Amphiprion sp.). Even tilapia (Oreochromis mossambicus) reared under brackish water conditions are susceptible.
This publication will review what is currently known about A. oscellatum and the resulting disease. Clinical diagnosis is straightforward, but management strategies may be quite different depending on the host species, the intended use of the fish (food versus ornamental), and the design of the culture system.
Description of Causative Organism
Amyloodinium is an extracellular parasite that also has a free-living phase. It attaches to its host using a rhizoid root-like structure that penetrates deep into host epithelium, causing substantial damage to tissue at the attachment site. It is an obligate parasite and requires a fish to both complete its life cycle and to survive for any extended period. It can also be propagated experimentally in the laboratory on gnobiotic guppies, Poecilia reticulatea (Noga and Bower, 1987), walking catfish (Clarias batrachus) gill (G1B) cells (Noga, 1987; Noga, 1992), or red drum fin cells (Oestmann and Lewis, 1996).
Both Amyloodinium and Piscinoodinium are dinoflagellates in the family Blastodiniphyceae (Levy and Noga, 2005). Most dinoflagellates are free-living, often photosynthetic, and serve as an important food source for planktivorous organisms. Of these two genera, only Amyloodinium causes disease in marine fish. To date, only one species has been identified in this genus, A. ocellatum. Recent genetic studies suggest that A. ocellatum isolates from diverse geographic origins and from different species of fish are all the same species (Levy et al., 2007), although some morphologic studies by others have suggested that multiple species might exist (Landsberg et al., 1994). Other authors have also observed differences in environmental tolerance, especially with regard to the range of salinity tolerated (typically greater in estuarine isolates). While Levy et al. (2007) acknowledged that there were “behavioral” differences between the five isolates they studied, genetically they all belonged to a single species. However, these researchers did not rule out the possibility that there could be subspecies or “strains” of A. ocellatum. While more isolates of Amyloodinium from various geographic and ecological regions will need to be examined to confirm that only a single species exists, the data at present strongly points to this conclusion. Most, if not all, marine and brackish water fish are susceptible to A. ocellatum if the environmental conditions are conducive to its reproduction. The disease has been particularly problematic for red drum, striped bass, and clownfish in aquaculture settings, and for many cage-reared marine food fish.
Life Cycle
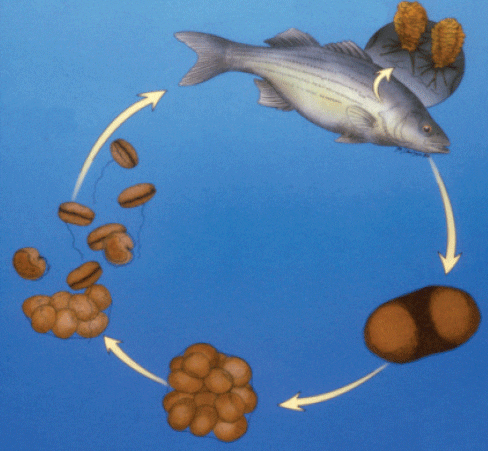
Amyloodinium ocellatum has a simple life cycle (meaning that no organism other than the fish needs to be present for the parasite to complete its life cycle). Having only one host allows the parasite to reproduce rapidly when the host fish are crowded. The parasite has three developmental stages (Fig. 1) that are superficially similar to those of the important marine parasite Cryptocaryon irritans, the cause of marine white spot disease. The three life stages are the trophont (the adult stage that feeds on the fish), the tomont (detached from the fish, this stage divides to form dinospores), and dinospores (the freeswimming stage that searches for and infects a host).
Note that a single trophont may produce as many as 256 infective dinospores (Brown, 1931; Nigrelli, 1936; Brown and Hovasse, 1946). Since the life cycle can be completed in as little as 3 to 6 days at 20 °C (Paperna, 1984a), the parasite load can increase rapidly and cause severe, acute mortality.
Only one life stage—the dinospore—is susceptible to drug treatment. Both the trophont (attached feeding stage) and tomont (encysted reproductive phase) are resistant. Thus, drugs used to control an outbreak must be directed at the dinospores. Treatment must be repeated multiple times or for a prolonged period to control the infection (see section on Treatment strategies below).
Environmental Tolerances
Amyloodinium ocellatum isolates tolerate a wide range of salinities and temperatures. Adverse conditions, such as suboptimal temperature or drug treatment, may stop tomont divisions but do not kill the parasite if the temperature or drug concentration is nonlethal to fish. The life cycle often may resume when the environment becomes more hospitable.
The temperature tolerance of A. ocellatum isolates seems to vary among isolates, but typically ranges from about 16 to 30 °C; salinity tolerance is also variable and has been reported as high as 50 ppt and as low as 12 ppt (Paperna, 1984a). The parasite cannot reproduce below 15 °C (Paperna, 1984a), but many susceptible fish species cannot be cultured (or may not even survive) at this low temperature. Noga and Levy (1995) cite an unpublished study in which tomonts exposed to low temperatures (< 15 °C for 17 weeks) were acclimated to 25 °C and continued to produce infective dinospores. Noga and Levy (1995) review work that suggests that salinity as low as 10 ppt may protect marine aquarium fish (Hojgaard, 1962), yet they note that outbreaks in wild fish have been reported at salinities as low as 2 to 3 ppt in estuarine waters of the Gulf of Mexico. These discrepancies may be explained by variations in tolerances between isolates and are not likely representative of the tolerances of all A. ocellatum.
Oestmann and Lewis (1996) made the serendipitous observation that magnesium may affect the survival of A. ocellatum in recirculating systems. Tomonts produced by trophonts cultured in vitro in magnesium-deficient salt water appeared normal but did not divide. The researchers speculated that it may be possible to inhibit the tomont’s reproduction with artificial sea water. It is not unusual for public aquaria to make their own salt mixes, so this strategy may be worthy of further investigation.
Transmission
Amyloodinium is transmitted through direct contact with live dinospores. Thus, the infection can be spread via water contaminated with live dinospores, including aerosolized droplets (Roberts-Thompson et al., 2006). Dinospores range in size from 12 to 15 ?m in diameter (Brown, 1931; Nigrelli, 1936; Landsberg et al., 1994). It is also likely that the parasite may be transmitted by fomites (nets, hands, shoes, equipment, etc.) that have contacted contaminated water. However, there is no evidence that dinospores are “sticky” like some other protozoan parasites.
Birds, wildlife or other terrestrial animals that might move between culture systems (including dogs that swim in different areas on a farm) might also transmit parasites via infected water or even by moving an infected fish into a non-infected area. Dead fish can also be a reservoir for Amyloodinium in that trophonts can drop off into the sediment and divide to form tomonts, or can even divide on the dead fish. For this reason, it is advisable to remove dead fish as quickly as possible from the system. If possible, the aquarium bottom should also be routinely siphoned to remove tomonts, helping to reduce the number of potentially infective dinospores (J. Landsberg, personal communication).
Minimizing the movement of parasites around a culture facility is a critical element in a preventive health program. This is discussed in more detail below, but the goal of foot baths, net rinses, and physical separation is ultimately to prevent the movement of these tiny, infectious, parasitic stages to areas where uninfected fish may be housed.
Potential Impact on Aquaculture Businesses
Amyloodinium can devastate aquacultural businesses, since outbreaks typically have an acute onset, spread very rapidly, and cause extensive mortalities. Farms that raise “high risk” species such as red drum and clownfish are strongly advised to implement strong biosecurity protocols and educate workers about the importance of keeping this organism out of the culture systems. Once established on a farm that raises sensitive species of fish, the parasite may prevent the business from being financially successful.
Potential Impact on Zoos and Aquaria
Zoos and aquaria do not usually maintain fish at the densities found on commercial aquaculture farms. Still, this parasite is quite problematic as it can infect a broad range of hosts. Essentially all teleosts should be considered susceptible. Elasmobranchs should also be considered at risk (Keller, 2006), though there is much less information and documentation about amyloodiniosis in these species. There is also the possibility that A. ocellatum may infect some parasitic invertebrates of fish. Colorni (1994) reported the organism as a hyperparasite of the monogenean Neobenedeinia melleni, an important parasite of marine fish in its own right, but this appears to be extremely rare.
Preventing the introduction of A. ocellatum into an established collection is the goal of quarantine programs (discussed in more detail below). Fish should be examined when they enter quarantine and periodically while in quarantine. Examination should include collecting biopsy specimens from gills, skin and fins. Any fish that dies in quarantine should be subjected to a thorough necropsy. Sea water brought in with new fish should NEVER be introduced into holding aquaria or exhibits. Polymerase chain reaction (PCR) testing (Levy et al., 2007) may be used to test fish or water for the presence of the organism, but unless there are specific concerns regarding a group of fish, careful quarantine, with monitoring and examination of gill and fin tissue with a light microscope (procedure described below), should be adequate in most zoological and aquarium settings.
Prophylactic freshwater dips for up to 5 minutes have been used to remove trophont stages for euryhaline (and potentially marine) species that can tolerate fresh water longer than A. ocellatum (J. Landsberg, personal communication).
Diagnosis
Disease Presentation
Morbidity and mortality from amyloodiniosis can be severe, sometimes with rapid onset over a period of a few days. Affected fish may die suddenly, showing few clinical signs, but in most cases behavioral and physical changes will be observed before death. If the primary site of infection is gill, which seems to be the most common, the primary clinical signs will be respiratory. These may include increased respiratory rate (rapid gilling and movement of the opercula), “piping,” and gathering at the surface or in areas with higher dissolved oxygen concentrations, as well as reduced appetite. If the primary site of infection is skin, infected fish sometimes develop a white or brown coloration (“velvet”) or cloudy appearance, which is most visible when viewed with indirect lighting such as a flashlight (Levy et al., 2007). Such fish may display signs of “flashing” or rubbing on tank walls, the substrate, or other structures in their environment. Again, feeding behavior likely will be poor and some fish may appear emaciated. Fish with Amyloodinium infection alone do not typically have ulcers, white spots, or fuzzy lesions, but the skin can seem “hazy” in appearance. If the infection is confined to the gill, the “velvet” appearance will not be present.
Preliminary Diagnosis
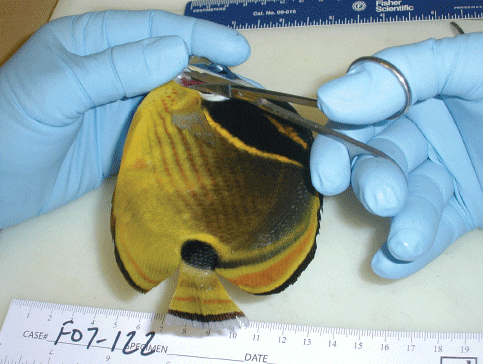
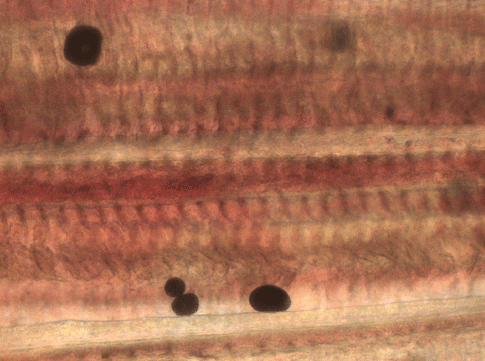
Clinical diagnosis of amyloodiniosis is straightforward, although less experienced examiners have confused A. ocellatum and Cryptocaryon irritans. Both are ectoparasites that are easily detected by microscopic examination of infected tissue. Gill or skin biopsies (Figs. 2 and 3) reveal the parasite attached to tissue. Both organisms are easily visible at total magnification of 40X and 100X with a light microscope. As shown in Figure 4, A. ocellatum is dark brown in color, ovoid to pear-shaped, and nonmotile (Brown, 1931; Nigrelli, 1936; Brown and Hovasse, 1946). In contrast, C. irritans is a large, ciliated protozoan and will be seen moving on or within tissue. Noga and Levy (1995) report that A. ocellatum trophonts can be up to 350 ?m, but smaller sizes of < 150 ?m are more typical (Alvarez-Pellitero, 2008). It is not unusual to have organisms of different sizes visible in one field. Because the parasite detaches from tissue exposed to fresh water, it is important that wet mounts be made using either sea water or, for estuarine fish, water of a similar salinity to the fish’s original water source.
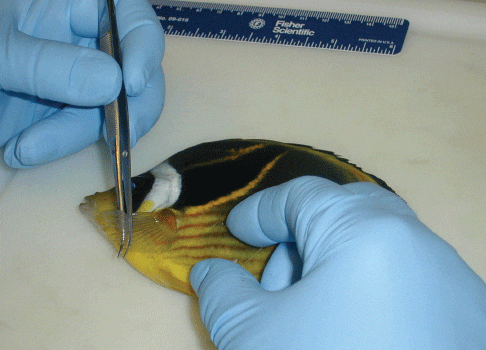
Carcasses submitted for microscopic examination should not be allowed to come into direct contact with ice during shipping or handling because parasites may fall off or lyse when exposed to water as the ice melts, causing the examination to have false negative results.
To detect the parasite microscopically, a fairly high number of organisms must be present. In fish with subclinical infections, the number of organisms present may be quite small and could be overlooked. A practical method of testing for Amyloodinium stages can be to immerse the dead fish (if it is not needed for other diagnostic tests) in a small amount of fresh water and allow for the trophonts or recently divided tomont stages to drop off. Water can be gently spun in a centrifuge or pipetted into a petri dish and then examined under a dissecting microscope for parasitic stages (J. Landsberg, personal communication).
Collecting Biopsy Samples
Collecting tissue from gills or skin for microscopic examination is a routine clinical procedure (Noga, 2010). Biopsy material may be collected from living fish in a nonlethal method, and tissue should always be collected as part of a necropsy examination. When working with live fish, it is best to collect biopsy samples before anesthetizing the fish. Anesthetics (most commonly tricaine methane sulfonate, also known as MS-222) might cause parasites to detach from the fish, leading to false negative results when tissue is examined with a light microscope, though it may be possible to recover the parasite from the bottom of the aquarium. Once tissues are prepared for examination they should be evaluated quickly. As samples begin to dry out, their diagnostic value diminishes rapidly.
A fish can be restrained by wedging it against the side of an aquarium or placing it on a flat, wet, non-abrasive surface. Covering the eyes may help quiet fish that are fully awake. If the procedure is performed as a non-lethal diagnostic test, it is important to take only the minimum amount of tissue needed to get a diagnostically useful sample. If the fish is too large to be handled easily and safely while awake, or it is otherwise intractable, chemical restraint (sedation or anesthesia) is appropriate. MS-222 is used to safely anesthetize many species of fish. Concentrations of 50 to 125 mg/L are often used to induce anesthesia, while lower concentrations may be used to maintain it or to simply sedate fish. MS-222 is acidic and should be buffered with 2 parts, by weight, of sodium bicarbonate (baking soda) to prevent pH from dropping when the chemical is added to the water. Using unbuffered tricaine has been shown to detach ectoparasites from fish (Callahan and Noga, 2002). If fish are intended for human consumption, be aware that the U.S. Food and Drug Administration (FDA) requires a 21-day withdrawal period for fish treated with MS-222, so they cannot be sent to slaughter for a full 21 days after exposure to the drug. Neiffer (2007) provides an excellent review of fish sedation and anesthesia.
To make a gill biopsy (Fig. 2), carefully lift the operculum of the fish and use a small pair of scissors (embroidery scissors work well and are inexpensive) to cut the tips of a few gill filaments and place them on a glass slide. To minimize the chance of causing hemorrhage, scissors should never be pointed down, as there is a major artery that runs along the base of the gill arch. A drop of water (water from the fish’s aquarium or water of a similar salinity) should be immediately placed on the gill tips, followed by a cover slip. The sample is now ready to be examined with a light microscope.
To make a mucus biopsy of skin on the body, simply run a coverslip along the back of the fish or under the pectoral fin (Fig. 3). If there are obvious abnormalities such as ulcerations or white spots, it is best to take a sample from the edge of the lesion or, in the case of a white spot, directly from the spot. When intended as a non-lethal examination, the amount of tissue (epithelium with scales) and mucus removed for examination should be minimal. If the procedure is part of a necropsy, taking a larger sample is acceptable. After making the scraping, the edge of the coverslip will have mucus, cells and scales, all barely visible to the naked eye and often appearing as brown mucoid material. This sample is placed on top of a drop of water (of the appropriate salinity) and examined with a light microscope as described above.
To make a biopsy of skin on the fins, a very small piece of fin tissue (usually from the edge of the caudal fin) can be collected with small scissors and placed on a cover slip. It is important to lay the tissue flat to maximize visibility. A drop of water (of appropriate salinity) is used to make the wet mount, and a cover slip is placed on top of the sample before microscopic examination.
The biopsy procedures described above are easy, quick and inexpensive. They are the best way to rapidly diagnose Amyloodinium and should be used routinely in any facility that houses or raises fish. The cost of a microscope for this test is relatively low compared to the expensive investment required by modern aquaculture, and it should be considered indispensible to a successful operation. Early diagnosis and control of a single outbreak will often more than pay for the cost of purchasing a microscope. Having trained personnel available to check fish also is a worthwhile investment.
Ancillary Diagnostic Tests
Histology. Amyloodinium ocellatum also can be diagnosed in samples of gill or skin collected for histological examination during necropsy. Tissues are typically placed in 10% neutral buffered formalin prior to histological processing. This method is less sensitive than examining wet mounts of infected tissue and is likely to detect only severe infestations, as at least some parasites will detach from the tissue, especially during fixation with formalin. Note that formalin has also been used to treat amyloodiniosis. Parasites that remain attached to tissue through fixation, processing and staining will be readily visible. Failure to find the organism using histologic methods does not guarantee that fish are not infected. Histologically, affected gills may appear hyperplastic (a proliferation of cells) and lamellar fusion may be evident (Paperna, 1980; Johnson, 1990).
Serology. Amyloodinium-infected fish can develop a specific, antibody-mediated response to the parasite. This has been described by Smith et al. (1992) in blue tilapia (Oreochromis aureus) and by Cobb et al. (1998a) in the tomato clown fish (Amphiprion frenatus). The specific immune response is typically delayed in fish, so serology is not suitable for detecting early infections, although it may have a place in identifying animals that have had past exposure to the parasite. The antibody response seems to be directed at the trophonts, but does not seem to inhibit attachment of dinospores (Cobb et al., 1998b).
Molecular methods. Recently, Levy et al. (2007) described a sensitive method of detecting a single parasite in sea water or gill tissue using a gene test (PCR or polymerase chain reaction). This procedure would be advantageous for detecting subclinical infections, or the presence of the parasite very early in the course of infection when few are present. This technology may be very useful for aquaculture operations that find it cost-effective to screen susceptible fish (or water) as part of a preventive medicine program. It is probably less cost-effective for detecting the occasional infection in ornamental or aquarium fish.
Treatment Strategies
Management decisions will be based on the species of fish infected, the intended use of these fish, and constraints that may be imposed by the type of system they are housed in. In general, treatment involves killing the free-swimming dinospores before they can attach to a new fish host or removing or killing the trophonts from the fish, thus breaking the life cycle. Because of the multistage life cycle, multiple treatments or extended treatments (> 10 to 14 days) will be necessary to completely control an outbreak of A. ocellatum.
Environmental conditions affect the speed at which the parasite completes its life cycle and the length of time that dinospores are infective. This has been reviewed by Johnson (1990). Understanding the timing of the life cycle is essential to knowing when repeated treatments may be necessary. Under optimum conditions, A. ocellatum should complete its life cycle in 12 days or less. Trophonts remain attached to fish for 2 to 5 days at 24 °C, although some individuals may detach within 24 hours. At 20 °C and 20 to 40 ppt salinity, the time between detachment and emergence of infective dinospores of a Red Sea isolate was 3 to 6 days (Paperna, 1984a). Dinospores may remain infective for at least 6 days (Bower et al., 1987), though infectivity falls off quickly after 24 hours. With these parameters in mind, it would seem prudent to repeat drug treatment daily unless using a compound that remains continuously active throughout the treatment period.
Because treatments for fish intended for human consumption are much more restricted than those for aquarium or exhibit fish, management of the parasite in these broad groups will be discussed separately.
Treatment Options for Food Fish
Several brands of formalin are approved for use in fish by the FDA. The efficacy of this parasiticide against A. ocellatum dinospores has been demonstrated. There is no required withdrawal period when formalin is used in fish intended for human consumption. Formalin concentrations as low as 25 mg/L, a common therapeutic dose, cause trophonts to fall off of parasitized fish within a few hours (Paperna, 1984b). However, tomonts are not killed, so infective dinospores will still emerge to infect the fish unless the fish are removed to an uncontaminated environment immediately after treatment.
Hydrogen peroxide is also FDA-approved for use in food fish as Perox-Aid, a 35% hydrogen peroxide solution. Montgomery-Brock et al. (2000) described the use of 35% hydrogen peroxide to treat mullet (Mugil cephalus) fry infested with Amyloodinium. Fish in a flow-through seawater system were treated once with 25 mg/L hydrogen peroxide for 30 minutes. Mortalities decreased from 200 to 1,000 fish per day to fewer than 10 per day within 3 days of treatment. The authors present data only for the first 4 days following treatment. Repeated treatments would be necessary under most culture conditions. Removing tomonts following treatment might be an important strategy when using this method (Noga, 2010).
Another option is using fresh water to kill infective, free-swimming dinospores and trophonts. Repeated dips in fresh water may help reduce the number of dinospores available to re-infect fish; however, unless fish can be moved into a completely freshwater system the disease will not be controlled because Amyloodinium can complete its life cycle in brackish water. It also appears that there are different strains of the parasite, some of which are less tolerant of low salinity (e.g., isolates from the Red Sea) and others that tolerate it (e.g., isolates from the Gulf of Mexico). Thus, the effectiveness of using fresh water may depend upon the particular isolate of Amyloodinium. For most isolates that affect stenohaline marine fish (such as most tropical reef species), reducing salinity to < 10 ppt will usually be sufficient, but fish must be kept at this low salinity for at least 2 to 3 weeks. This strategy may not be as efficacious in euryhaline fish from estuarine environments. While not yet verified, it is possible that trophonts remaining on the fish may survive the hyposalinity treatment and may be able to resume their life cycle when environmental conditions became more favorable.
Copper sulfate was first reported as a treatment for A. ocellatum by Dempster (1955). It is toxic to dinospores and has been a standard treatment for years. It is not currently FDA-approved for controlling amyloodiniosis, but has been approved for a long time as an algicide by the U.S. Environmental Protection Agency (EPA). Consequently, copper sulfate has been used in food fish aquaculture for many years, and the FDA has not formally objected to this practice. Efforts are underway to gain FDA approval of copper for use in aquaculture. Coppercontaining compounds are dangerous to use because the margin of safety is quite low. This means that the difference between the amount of chemical required to kill the parasite and the amount of chemical that will kill the fish is small. Cardeilhac and Whitaker (1988) reviewed the use of copper in marine systems, where copper chemistry is complex. The desired therapeutic concentration is 0.15 to 0.2 mg/L free copper ion. Maintaining this concentration constantly ensures that a lethal dose of copper would always be present as each individual organism develops into a dinospore. Free copper ion does not stay in solution for a long time, so copper concentrations should be measured at least once each day—twice each day if possible— so that additional copper sulfate can be added when necessary. Treatment with copper-containing compounds should be continued for at least 2 to 3 weeks to control an outbreak of A. ocellatum, with the goal of maintaining a continuous concentration of 0.15 to 0.2 mg/L free copper ion. When treatment fails it is often because monitoring was inadequate and additional copper sulfate was not added to maintain the proper concentration. Surviving fish can be biopsied as described above to ensure that the parasite has been effectively controlled. Coppercontaining compounds are highly toxic to invertebrates and should never be used in systems housing these animals.
Treatment Options for Ornamental Aquarium Fish
All of the treatment options mentioned above can be used with marine ornamental fish. Copper is most commonly used, especially in quarantine systems. Copper is highly toxic to invertebrates. Elasmobranchs are also much more sensitive to copper compounds than teleosts, and copper should not be used in exhibits housing elasmobranchs.
Chloroquine is an anti-malarial compound that has proven to be safe and effective in controlling A. ocellatum in marine aquarium fish (C. Bower, personal communication cited in Noga, 2010). While it is routinely used in the industry, it cannot legally be used in any fish intended for human consumption or categorized as a food fish by FDA. However, it has been an excellent option for the ornamental marine fish industry, especially for use in clown fish production and as part of quarantine protocols of public aquaria. It can also be useful in retail outlets selling marine aquarium fish, though it is expensive. It is effective in the control of A. ocellatum at concentrations of 10 mg/L, but the system may need to be re-dosed after 7 or 8 days (Lewis et al., 1988). Infected fish should be monitored with gill and skin biopsies to determine whether and when re-dosing is needed. Treated water should be run through an activated carbon filter to remove any residual chemical prior to discharge.
Role of the Rearing System in Treatment Protocols
When marine fish are reared in re-circulating systems, chemical filtration (activated carbon) should be taken off-line during drug treatment. Activated carbon will remove the drug, resulting in treatment failure. If a flow-through system must be treated, a continuous drip may be needed to maintain appropriate levels of chemical in solution. This can be labor-intensive, but is worth the effort to have a successful outcome. In this situation, the fish culturist must be mindful of where the effluent from the system goes. A carbon filter may be needed to remove the treatment chemical before water leaves the premises.
An outbreak of A. ocellatum in cage culture would likely be especially challenging. Intermittent treatments probably would be required, as continual treatment would be difficult or impossible. Cages would have to be covered during treatment to maintain chemical concentrations for necessary time periods, and chemical selection would be limited by environmental regulations. A certain amount of trial and error might be required to develop an effective treatment protocol in these circumstances.
Preventing Infection
Fish that survive an amyloodiniosis outbreak can become immune (Smith et al., 1992; Cobb et al., 1998a; Cobb et al., 1998b), which can facilitate recovery from an outbreak. However, recovered fish are not necessarily free of parasites so if na?ve fish are introduced another outbreak is likely. Preventing the introduction of the parasite in the first place is extremely important for a successful aquaculture operation.
Disinfecting water with ultraviolet (UV) irradiation or ozone can kill dinospores and may be especially useful in re-circulating systems. Both methods are used routinely in commercial aquaculture and public aquaria. UV units are also becoming more common in home aquaria. Ozone disinfection in home aquaria is not common at this time, though some small units are being marketed. Ozone is not recommended for any but the most experienced aquarists.
Avoiding Potential Sources of Infection
The most common source of Amyloodinium is infected fish or water. Keeping infected fish out of aquaculture systems is accomplished using effective quarantine protocols, which are described below. Introduced fish should be transferred into facility water as part of the quarantine protocol. Water from an outside source may contain dinospores and should never be introduced into a facility.
Infective dinospores could be transported in aerosolized water droplets (Roberts-Thompson et al., 2006). Droplets from static systems were shown to be transmissible for up to 0.44 m (1.44 feet); however, droplets from dynamic ones transferred up to 3 m (9.8 feet). This means that adjacent aquaria and possibly even ponds could spread the infection. Thus, the quarantine area should be as geographically separated as possible from production units or exhibit areas. If aquaria must be situated close to each other, they should be covered.
Abreu et al. (2005) described recurrent infections of Brazilian flounder (Paralichthys orbignyanus) with A. ocellatum. They claimed that there were high numbers of tomonts in biofilms from infected systems and hypothesized that these served as the source of the recurrent infections. However, they did not use any specific gene test to prove that what they were counting were truly A. ocellatum parasites. None the less, scrubbing the bottom of infested aquaria with a brush that removed the biofilm, followed by treating the contaminated surfaces with 30% hydrochloric acid solution, prevented the recurrence of the disease for up to 1 month. Affected fish were fed wild-caught fish (treated for Amyloodinium before introduction to the system) and chopped frozen fish. Incoming sea water was filtered through two sand filters. Amyloodinium may have been able to gain entry into the culture system via either the food or in-coming water, causing the recurring outbreaks.
We are aware of one outbreak of A. ocellatum in red drum that seemed to have been introduced via feed. Red drum broodstock were fed wild-caught pinfish (Lagodon rhomboides) that were being stored in a home freezer. Amyloodinium trophonts were readily visible on gill biopsies of pin fish when they were examined with a microscope. It is not known how long, or at what temperature, infective material would need to be frozen to prevent this from being a risk factor. Since cryopreservation of A. ocellatum has not been demonstrated, it may be that there was another source of infection that was never identified. Tomonts have been observed in intestinal contents of infected red drum, so it may be that trophonts can drop off of gills, pass through the gastrointestinal tract, and remain viable (J. Landsberg, personal communication).
Quarantine and Biosecurity Protocols
Quarantine, by definition, implies isolation and seclusion. In all aquaculture systems, a period of quarantine should be required for all new arrivals into a facility. This period of time allows the fish to acclimate to the new environment and recover from transport. It also ensures as much as possible that they are not carrying an infectious disease that might put the established population at risk. Actually, quarantine reduces only the likelihood that an epidemic will occur in the system to which they are introduced (as it will more likely occur in quarantine). Quarantine itself does not prevent the introduction of a pathogen. However, screening for and removing subclinically infected fish does reduce the likelihood of introducing a pathogen (although that does not ensure its exclusion). Whitaker (1999) presents general principals and a generic outline for quarantine of newly arrived fish for zoos and public aquaria. Although 30 days is often suggested as a minimum quarantine period, this may not be sufficient to prevent the introduction of A. ocellatum. As a precaution, some hatcheries culturing marine and estuarine animals subject their broodstock to a freshwater dip before introducing them into the hatchery. Screening fish during quarantine for A. ocellatum and other parasites is easily done using the biopsy protocols previously mentioned. Biopsies especially should be performed on sick fish (e.g., those which have poor appetite, appear to be listless, are abnormally dark in color, or are seen flashing or exhibiting rapid respiration rates). Necropsies should be performed on fish that die during quarantine. Clownfish and red drum are particularly susceptible to A. ocellatum and extra vigilance is recommended when working with these species. These highly susceptible species may be excellent candidates for the PCR screening described by Levy et al. (2007).
Most quarantine protocols for marine fish include a routine copper treatment, which is typically continuous for 21 to 30 days. This would be done at the 0.15 to 0.2 mg/L free copper ion concentration as previously described. This treatment should eliminate A. ocellatum from fish while in quarantine, but biopsies should still be used to ensure that fish are not harboring parasites. Poor efficacy, or treatment failure, when using copper-containing compounds in marine systems is usually associated with inadequate monitoring of the free copper ion concentration to be sure it does not fall below the required therapeutic level. This allows some parasites to evade treatment and can result in an outbreak several weeks after the copper treatment has been discontinued. Freshwater dips may also be useful in eliminating dinospores and trophonts from fish.
Biosecurity is defined as protection from biological harm. Although part of quarantine, biosecurity is broader in concept and applies to an entire facility, not just a quarantine area. Good biosecurity protocols often start with record keeping, for example the source of animals, when they were received, the number received, examinations done as part of quarantine, where they go when they are released from quarantine, and follow-up once they are in production or on display. In addition, biosecurity plans may include the restriction of personnel, the use of dedicated equipment at different points in a facility, the physical separation of animal units (i.e., quarantine ideally should take place in a completely separate area or building from production or exhibit units), and disinfection protocols for tools (dip nets, equipment) and people (the use of footbaths between rooms or buildings) (see SRAC publication 4703).
Disinfection Protocols
Freshwater dips of 1 to 3 minutes cause A. ocellatum trophonts to detach from infected fish (Bower et al., 1987). It is unclear whether this is sufficient to actually kill them, however, and given the tolerance of trophonts and tomonts to environmental conditions, it may not be prudent to assume that this would happen. Noga and Levy (1995) also suggest that while freshwater dips may dislodge most trophonts, they may not dislodge all of them. Freshwater dips alone are probably not sufficient to prevent the movement of this important parasite around an aquaculture facility. However, testing each species for freshwater tolerance and monitoring for tomonts has been effective in some situations (J. Landsberg, personal communication).
Noga and Levy (1995) have reviewed a number of drugs that have been used in efforts to disinfect systems contaminated with A. ocellatum. Of these, benzalkonium chloride seems most promising for use as a routine disinfectant in aquaculture or aquarium facilities, although the compound is not FDA-approved for use in fish. This chemical is in the group of disinfectants known as quaternary ammonium compounds.
Ultraviolet (UV) light is an important component of disinfection and sanitation protocols in many aquaculture facilities and public aquaria. The efficacy of UV light is based on several factors, including water clarity, water flow rate, exposure time of the pathogen, and bulb wattage. Because the amount of water passing in proximity to a bulb at any given time is small compared to the total volume of flow, UV bulbs are typically set up on a “side-stream” off the regular line. Consultation with an experienced manufacturer or sales representative is recommended to ensure proper sizing and maintenance of bulbs, as well as optimal flow adjustment through the UV unit.
Ozone (O3), is an unstable molecule that oxidizes organic material indiscriminately. It is used to disinfect and sanitize aquarium water and is used in aquaculture facilities to minimize the number of infectious particles (parasitic, bacterial, fungal, etc.) in water that may pose a risk to cultured fish. Some small ozone units are marketed to advanced home aquarists, but their use in home systems is not common. Ozone can be hazardous to personnel who come into contact with the gas, and it is lethal to fish if free ozone is accidentally released into a unit that houses live animals. Consultation with an experienced manufacturer or sales representative is strongly recommended to ensure proper sizing and plumbing of an ozone unit.
Summary
Amyloodinium ocellatum is a common and important parasite of warmwater marine and brackish water fish. All fish in such environments should be considered susceptible. The organism attaches to external epithelial surfaces, including gills and skin. It is easily observed on wet mounts of infected tissue using a light microscope (Fig. 4). The parasite reproduces rapidly because of its simple (direct) life cycle. The attached, feeding stage (trophont) may be difficult to treat and the encysted, reproductive phase (tomont) is completely resistant to drug treatment. The free-swimming dinospores, released from the tomont, are susceptible to drug treatment, but they emerge from tomonts at different times and may live for up to several days. Repeated treatments (daily is probably best) or continuous treatments for 2 to 3 weeks are usually required to control an outbreak. Some drugs may cause trophonts to fall off fish; however, they usually remain viable so live dinospores are released if treatment protocols are not adhered to.
Formalin and hydrogen peroxide are both FDAapproved for use in food fish in the U.S. It is hoped that the FDA will also approve copper-containing compounds. Chloroquine is effective in marine aquarium fish and retail pet stores but must never be used in food fish. Freshwater dips can be very useful if the fish is able to tolerate freshwater conditions for a longer period of time than the trophonts.
Quarantine and biosecurity are essential to preventing recurring problems with A. ocellatum in mariculture facilities. The parasite is easily introduced with infected fish or water, and once in a facility, it may be spread by aerosolization, fomites, or subclinically infected fish. Disinfection protocols should be set up to help prevent accidental introduction or movement of infective material around a facility.



