Introduction
Ammonia causes stress and damages gills and other tissues, even in small amounts. Fish exposed to low levels of ammonia over time are more susceptible to bacterial infections, have poor growth, and will not tolerate routine handling as well as they otherwise would. Ammonia is a killer when present in higher concentrations, and many unexplained production losses have likely been caused by ammonia.
Ammonia accumulates easily in aquatic systems because it is a natural byproduct of fish metabolism. All animals excrete some waste in the process of metabolizing food into the energy, nutrients, and proteins they use for survival and growth. In fish, the principal metabolic waste product is ammonia. Because it is continuously excreted and potentially lethal, successful aquaculture operations must therefore incorporate methods to detect and eliminate ammonia before it can accumulate and harm fish.
A byproduct of protein metabolism, ammonia is primarily excreted across the gill membranes, with only a small amount excreted in the urine. The decay of uneaten feed and organic matter create small amounts of ammonia, but in most aquaculture systems, fish themselves are the primary source of the compound. The more feed a fish receives, the more ammonia it will produce. However, even a starved fish will produce some ammonia.
Ammonia may be present in city or well water. Even trace amounts can be toxic to fish, and ammonia is colorless, and, in small amounts, odorless. Therefore, the only way for an aquarist or producer to know if ammonia is present is to test the water.
In water, ammonia occurs in two forms, which together are called total ammonia nitrogen, or TAN. Chemically, these two forms are represented as NH4+ and NH3. NH4+ is called ionized ammonia because it has a positive electrical charge, and NH3 is called un-ionized ammonia (UIA) because it has no charge. This difference is important to know because NH3, un-ionized ammonia, is the form more toxic to fish. Both water temperature and pH affect which form of ammonia is predominant at any given time in an aquatic system.
The Nitrogen Cycle
A biological process called the nitrogen cycle eliminates ammonia from the water by converting it to other, less toxic compounds (Figure 1). The ammonia fish excrete is converted to a compound called nitrite (NO2-) by several genera of bacteria, including Nitrosospira and Nitrosomonas. Other groups of bacteria, including Nitrospira and Nitrobacter, convert nitrite to nitrate (NO3-).
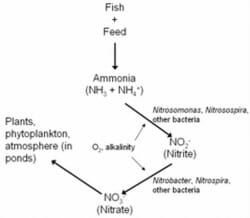
Figure 1. The nitrogen cycle. Nitrifying bacteria use oxygen and alkalinity to convert ammonia and nitrite into the less toxic byproduct, nitrate, which is then used by plants or returned to the atmosphere.
In ponds, this process takes place in the surface layers of the mud, and on plants or other structures. In tanks or aquaria, a biological filter, or biofilter, must be provided as a place where the bacteria can live and flourish. A new biofilter requires six to eight weeks to build up sufficient bacteria to effectively reduce ammonia and nitrite levels.
Other important points to mention about the nitrogen cycle are that both groups of nitrifying bacteria need oxygen and alkalinity to function. If oxygen levels are not sufficient, the process can break down, and ammonia and nitrite levels will increase. Alkalinity (bicarbonate and carbonate) is also used by the nitrifying bacteria. If alkalinity is less than 20 mg/L, the nitrifying bacteria will not be able to function.
It's also important to note that nitrite is toxic to fish at levels as low as 0.10 mg/L. If the biofilter is immature or impaired, adding chloride in the form of salt (sodium chloride) or calcium chloride at the rate of 10 mg/L chloride for each 1 mg/L nitrite will reduce the toxic effects of nitrite on fish.
Nitrate, the end product of the nitrogen cycle, is considered to be harmless to fish in natural systems and ponds as it is used as a fertilizer by plants, including phytoplankton. In closed systems with little or no water exchange, however, nitrate will accumulate and may be harmful if higher than 250 mg/L.
Ammonia Testing
All aquaculturists and hobbyists should invest in a water quality test kit. A good water quality management program will reduce fish disease problems, promote growth, and lessen the need for chemical treatments. A water quality test kit will pay for itself many times over, both in numbers of fish saved and increased production.
Most commercial ammonia test kits measure the total ammonia nitrogen (TAN). Again, it is the un-ionized ammonia (or UIA) portion of the TAN that is more toxic. The UIA fraction of the total TAN can be determined from the TAN measurement if you know the temperature and pH of the water. At high temperatures and high pH, there is more UIA. Therefore, a good ammonia test kit will include a TAN test, a pH test, and a thermometer.
There are two types of ammonia test kits, and each uses a different testing method to determine TAN. One is the Nessler's method and the other is the ammonia salicylate method. If formalin or formalin-containing products have been used within 24-72 hours to treat fish for parasites, the Nessler's method will result in a falsely elevated ammonia reading. Use of ammonia binding products will also cause false high ammonia readings with the Nessler's method. The reagent used in the Nessler's method contains a small amount of mercury that in many states must be disposed of as hazardous waste.
The other testing method is the ammonia salicylate method. This method is not affected by ammonia binding products or formalin treatments. The ammonia salicylate method is also more accurate than the Nessler's method when testing ammonia in seawater, and it does not require disposal of a hazardous waste.
When Should Ammonia Be Tested?
If stocking densities are high, ammonia should be tested every 10 to 14 days in ponds, and at least once a week in tanks. If multiple tanks depend upon a common biofilter (i.e., a recirculating system), there is no need to check every tank individually. Keep records for all tests, and whenever ammonia is found, increase the frequency of testing until the problem is corrected. Whenever fish are sick, test the water quality.
Ammonia is responsible for more unexplained losses in aquaculture than any other water quality parameter. As previously mentioned, it is colorless and odorless, so the only way to know if it is present is to test for it. Fish submitted to a diagnostic laboratory are tested for diseases (bacteria, parasites, fungi or viruses) only. It is the responsibility of aquarists and producers to test the water quality, which is very likely to be the underlying problem.
Interpreting the Ammonia Test

Figure 2.

Figure 3
In healthy ponds and tanks, ammonia levels should always be zero. Presence of ammonia is an indication that the system is out of balance. Therefore, any ammonia in a pond or tank should alert the producer to start corrective measures. Un-ionized ammonia (UIA) is about 100 times more toxic to fish than ionized ammonia.
This UIA toxicity begins as low as 0.05 mg/L, so the result of the TAN test needs to be further calculated to find the actual concentration of UIA. To do this calculation, the temperature and pH need to be measured. Once the pH and temperature are known, the fraction of UIA can be calculated using a multiplication factor found in Table 1. Find the temperature on the top row of the table, and the pH in the left column. The number at which the appropriate column and row intersect in the table is multiplied by the TAN to give the UIA in mg/L (ppm).
This calculation is summarized in Figure 2 and an example is given in Figure 3. Anytime the UIA is higher than 0.05 mg/L, the fish are being damaged. As the concentration rises above 0.05 mg/L, it causes more and more damage. At 2.0 mg/L, the fish will die. Again, any ammonia indicates a problem in your system. If you find it, take corrective measures immediately.
The first thing to do when ammonia is present in a pond or tank is to reduce or eliminate feeding. Fish are not likely to eat during periods of ammonia stress and the uneaten feed will only make the situation worse. Overfeeding is a major cause of high ammonia concentrations, and stopping the feeding will allow the natural nitrogen cycle to "catch up" with the nutrient load. If at all possible, a 25 per cent to 50 per cent water change will help to remove some of the ammonia. This is only feasible in small ponds or tanks, so don't try to solve an ammonia problem in a large pond by this method.
Low levels of dissolved oxygen limit the ability of nitrifying bacteria to convert ammonia and nitrite, so it is important to monitor dissolved oxygen.
In ponds, the addition of a phosphate fertilizer may help to relieve high TAN levels over a period of days by stimulating phytoplankton growth, which helps remove ammonia from the system; however, it may not help quickly enough in an acute ammonia crisis. Use a 0–20–0 fertilizer at a rate of 40 pounds per acre. It is important not to use a fertilizer that contains nitrogen because nitrogen will add to the problem. If phosphorus is not a limiting factor for algal growth in the pond, the phosphate fertilizer method will not work at all.
In tanks without a biofilter, the producer or aquarist should consider incorporating one. Given the six to eight weeks necessary to establish a biofilter, this will not help in a crisis, but it is a long-term solution to the problem.
In the short term, water changes and the use of ammonia binding products will alleviate ammonia toxicity. It's important to remember that these are short-term solutions. For long-term management, it's best to establish a biofilter.
Some chemicals used to treat diseases in fish, especially antibiotics, can be detrimental to the nitrifying bacteria in the biofilter. Both ammonia and nitrite levels should be tested more frequently after applying a disease treatment, to ensure that the biofilter is still functioning.
Summary
Ammonia is a major waste product of fish and the breakdown of feed and other organics. It can accumulate in aquaculture or aquarium systems, where it will, at the very least, decrease production. It is frequently a stressor that leads to disease, and in other cases it kills fish directly. The only way to detect its presence is to test for it. A fish farmer or aquarist should invest in a water quality test kit, learn how it works, and use it regularly.
Ammonia test kits only measure the total ammonia nitrogen (TAN). When this test indicates a reading above zero, producers or aquarists can determine the fraction of toxic un-ionized ammonia (UIA) after measuring pH and temperature. The multiplication factors are found in Table 1, and an example calculation is found in Figure 3.
When ammonia is present, the fish in the system should not be fed until the problem is corrected. In small systems, a water change will help, and in large ponds, a 0–20–0 fertilizer may help.
Test for ammonia regularly and take corrective measures as soon as you detect it. Severe problems may occur when tests are not performed frequently enough. Once fish have started to die, it is difficult to correct an ammonia problem without losing more fish.
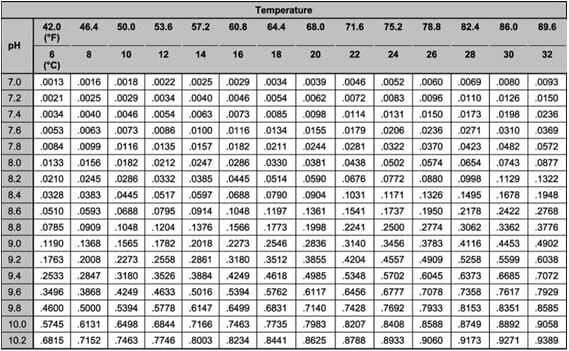
Table 1. Fraction of un-ionized ammonia in aqueous solution at different pH values and temperatures. Calculated from data in Emmerson et al. (1975). To calculate the amount of un-ionized ammonia present, the Total Ammonia Nitrogen (TAN) must be multiplied by the appropriate factor selected from this table using the pH and temperature from your water sample. See the example in Figure 3.
August 2009

