Water's Chemical Factors
Photosynthesis
Photosynthesis is one of the most important biological activities in standing pond aquaculture. Many water quality parameters such as dissolved oxygen, carbon dioxide, pH cycles, nitrogenous waste products are regulated by the photosynthetic reaction in phytoplankton. Simply stated, photosynthesis is the process by which phytoplankton uses sunlight to convert carbon dioxide into a food source and to release oxygen as a by-product (Figure 5).
| Figure 5. Equation illustrating the photosynthetic process which occurs in fish ponds and produces food for phytoplankton and releases oxygen as a by-product. |
In addition to supplying oxygen in fish ponds, photosynthesis also removes several forms of nitrogenous wastes, such as ammonia, nitrates, and urea.
The phytoplanktonic plant pigments involved in this chemical reaction are referred to as chlorophyll. These are the same pigments found in higher plants such as tree leaves.
Because the photosynthetic process is driven by sunlight, greatest concentrations of oxygen occur when the sun is highest on the horizon (usually 2-3 p.m. in the afternoon). At night, photosynthesis ceases and the phytoplankton primarily respirate.
Respiration is the reverse of photosynthesis in that oxygen is used by phytoplankton to convert food to energy and carbon dioxide is released as a by-product. Phytoplankton respiration also occurs during the day but fortunately for the fish farmer, there is usually a surplus of oxygen produced to compensate for the loss due to respiration. An exception is during extended periods of cloud cover, Respiration occurring in the absence of photosynthesis causes oxygen levels to decrease throughout the night. As a result the lowest concentrations of oxygen are observed immediately prior to sunrise.
Dissolved Gases
Dissolved gases are those which are in a water solution. An example of gas dissolved in solution is soda water which has large quantities of dissolved carbon dioxide. The most common gases are oxygen, carbon dioxide, nitrogen, and ammonia. Concentrations are measured in parts per million (ppm) or milligrams per liter (mg/1), both units of measure are the same. (One ppm or mg/1 is the same as one pound added to 999,999 pounds to total 1,000,000 pounds).
Oxygen
Dissolved oxygen (DO) is by far the most important chemical parameter in aquaculture. Low-dissolved oxygen levels are responsible for more fish kills, either directly or indirectly, than all other problems combined. Like humans, fish require oxygen for respiration. The amount of oxygen consumed by the fish is a function of its size, feeding rate, activity level, and temperature. Small fish consume more oxygen than do large fish because of their higher metabolic rate. Meade (1974) determined that the oxygen consumption of salmon reared at 57 oF was 0.002 pounds per pound of fish per day. Lewis et al. (1981) determined that striped bass raised at 77 oF consumed 0.012-0.020 pounds per pound of fish per day. The higher oxygen requirement by striped bass may be attributed to the statement that the metabolic rate doubles for each 18o F increase in temperature.
The amount of oxygen that can be dissolved in water decreases at higher temperatures and decreases with increases in altitudes and salinites (Table 2).
|
Table 2. Solubility of oxygen (ppm) in water at various water temperatures, salinities, and altitudes.
|
|||||
| Variable |
Temperature (oF) |
||||
|---|---|---|---|---|---|
| 68.0 | 71.6 | 78.8 | 82.4 | 86.0 | |
| Salinity (ppm) | |||||
| 0 | 9.2 | 8.8 | 8.2 | 7.9 | 7.6 |
| 5,000 | 8.7 | 8.4 | 7.8 | 7.5 | 7.3 |
| 10,000 | 8.3 | 8.0 | 7.4 | 7.1 | 6.9 |
| Altitude (ft) | |||||
| 0 (Sea Level) | 9.2 | 8.8 | 8.2 | 7.9 | 7.6 |
| 1,000 | 8.8 | 8.5 | 7.9 | 7.6 | 7.4 |
| 2,000 | 78.5 | 8.2 | 7.6 | 7.3 | 7.1 |
At sea level and zero salinity 68.0F water can hold 9.2 ppm, while at 86.0F, saturation is at 7.6 ppm. In combining this relationship of decreased solubility with increasing temperatures, it can be seen why oxygen depletion are so common in the summer when higher water temperatures occur.
Fish farmer, in an attempt to maximize production, stock greater amounts of fish in a given body of water than found in nature. At times during summer it may be necessary to supply supplemental aeration to maintain adequate levels of dissolved oxygen. Whereas in recirculation systems the farmer must supply 100 percent of the oxygen needed for the fish and beneficial nitrifying bacteria.
To obtain good growth, fish must be cultured at optimum levels of dissolved oxygen. A good rule of thumb is to maintain DO levels at saturation or at least 5 ppm (Figure 6). Dissolved oxygen levels less than 55 ppm can place undue stress on the fish, and levels less than 2 ppm will result in death (possibly 3 ppm for hybrid striped bass and yellow perch). Some warmwater species such as tilapia and carp are better adapted to withstand occasional low DO levels, while most coolwater species cannot.
| Figure 6. General dissolved oxygen requirements in parts per million (ppm) for fish. |
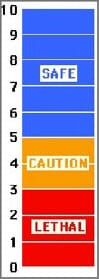 |
Fish are not the only consumers of oxygen in aquaculture systems; bacteria, phytoplankton, and zooplankton consume large quantities of oxygen as well. Decomposition of organic materials (algae, bacteria, and fish wastes) is the single greatest consumer of oxygen in aquaculture systems. Problems encountered from water recirculating systems usually stem from excessive ammonia production in fish wastes. Consumption of oxygen by nitrifying bacteria that break down toxic ammonia to non-toxic forms depends on the amount of ammonia entering the system. Meade (1974) determined that 4.0-4.6 pounds of oxygen are needed to oxidize every pound of ammonia. However, since other bacteria are present in pond and tank culture, a ratio of 6 pounds of oxygen to 1 pound of ammonia is recommended.
Oxygen enters the water primarily through direct diffusion at the air-water interface and through plant photosynthesis. Direct diffusion is relatively insignificant unless there is considerable wind and wave action. Several forms of mechanical aeration are available to the fish farmer. The general categories are:
- Paddlewheels
- Agitators
- Vertical sprayers
- Impellers
- Airlift pumps
- Venturia pumps
- Liquid oxygen injection
- Air diffusers

Mechanical aeration can also increase dissolved oxygen levels. Because of the lack of photosynthesis in indoor water recirculating systems, mechanical means of aeration is the only alternative for supplying oxygen to animals cultured in these systems. Oxygen depletions can be calculated, but predictions can be misleading and should never be substituted for actual measurements.
Carbon Dioxide
Carbon dioxide (CO2) is commonly found in water from photosynthesis or water sources originating from limestone bearing rock. Fish can tolerate concentrations of 10 ppm provided dissolved oxygen concentrations are high. Water supporting good fish populations normally contain less than 5 ppm of free carbon dioxide. In water used for intensive pond fish culture, carbon dioxide levels may fluctuate from 0 ppm in the afternoon to 5-15 ppm at daybreak. While in recirculating systems carbon dioxide levels may regularly exceed 20 ppm. Excessively high levels of carbon dioxide (greater than 20 ppm) may interfere with the oxygen utilization by the fish.
There are two common ways to remove free carbon dioxide. First, with well or spring water from limestone bearing rocks, aeration can "blow" off the excess gas. The second option is to add some type of carbonate buffering material such as calcium carbonate (CaCO3) or sodium bicarbonate (Na2CO3). Such additions will initially remove all free carbon dioxide and store it in reserve as bicarbonate and carbonate buffers. This concept is discussed in further detail under alkalinity.
Nitrogen
Dissolved gases, especially nitrogen, are usually measured in terms of "percent saturation." Any value greater than the amount of gas the water normally holds at a given temperature constitutes supersaturation. A gas supersaturation level above 110% is usually considered problematic.
Gas bubble disease is a symptom of gas supersaturation. The signs of gas bubble disease can vary. Bubbles may reach the heart or brain, and fish die without any visible external signs. Other symptoms may be bubbles just under the surface of the skin, in the eyes, or between the fin rays. Treatment of gas bubble disease involves sufficient aeration to decrease the gas concentration to saturation or below.
Ammonia
Fish excrete ammonia and lesser amounts of urea into the water as wastes. Two forms of ammonia occur in aquaculture systems, ionized and un-ionized. The un-ionized form of ammonia (NH3) is extremely toxic while the ionized form (NH4+) is not. Both forms are grouped together as "total ammonia." Through biological processes, toxic ammonia can be degraded to harmless nitrates (Figure 7).
| Figure 7. Equation illustrating how toxic un-ionized ammonia (NH3) is removed from water through the biological process called nitrification. | |||||
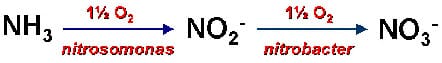 |
|||||
In natural waters, such as lakes, ammonia may never reach dangerous high levels because of the low densities of fish, But the fish farmer must maintain high densities of fish and, therefore, runs the risk of ammonia toxicity. Un-ionized ammonia levels rise as temperature and pH increase (Table 3).
|
Table 3. Percentage of total ammonia that is un-ionized at various temperatures and pH.
|
||||||
| pH | 54oF | 62oF | 68oF | 75oF | 82oF | 90oF |
|---|---|---|---|---|---|---|
| 7.0 | 0.2 | 0.3 | 0.4 | 0.5 | 0.7 | 1.0 |
| 7.4 | 0.5 | 0.7 | 1.0 | 1.3 | 1.7 | 2.4 |
| 7.8 | 1.4 | 1.8 | 2.5 | 3.2 | 4.2 | 5.7 |
| 8.2 | 3.3 | 4.5 | 5.9 | 7.7 | 11.0 | 13.2 |
| 8.6 | 7.9 | 10.6 | 13.7 | 17.3 | 21.8 | 27.7 |
| 9.0 | 17.8 | 22.9 | 28.5 | 34.4 | 41.2 | 49.0 |
| 9.2 | 35.2 | 42.7 | 50.0 | 56.9 | 63.8 | 70.8 |
| 9.6 | 57.7 | 65.2 | 71.5 | 76.8 | 81.6 | 85.9 |
| 10.0 | 68.4 | 74.8 | 79.9 | 84.0 | 87.5 | 90.6 |
| To determine un-ionized ammonia concentration, multiply total ammonia concentration by the percentage which is closest to the observed temperature and pH of the water sample. For example, a total ammonia concentration of 5 ppm at pH 9 and 680 F would be: 5 ppm total ammonia X 28.5% = 1.43 ppm. | ||||||
Toxicity levels for un-ionized ammonia depend on the individual species; however, levels below 0.02 ppm are considered safe. Dangerously high ammonia concentrations are usually limited to water recirculation system or hauling tanks where water is continually recycled and in pond culture after phytoplankton die-offs. However, the intermediate form of ammonia--nitrite--has been known to occur at toxic levels (brown-blood disease) in fish ponds.
Buffering Systems
A buffering system to avoid wide swings in pH is essential in aquaculture. Without some means of storing carbon dioxide released from plant and animal respiration, pH levels may fluctuate in ponds from approximately 4-5 to over 10 during the day. In recirculating systems constant fish respiration can raise carbon dioxide levels high enough to interfere with oxygen intake by fish, in addition to lowering the pH of the water.
pH
The quantity of hydrogen ions (H+) in water will determine if it is acidic or basic. The scale for measuring the degree of acidity is called the pH scale, which ranges from 1 to 14. A value of 7 is considered neutral, neither acidic or basic; values below 7 are considered acidic; above 7, basic. The acceptable range for fish culture is normally between pH 6.5-9.0.
Alkalinity
Alkalinity is the capacity of water to neutralize acids without an increase in pH. This parameter is a measure of the bases, bicarbonates (HCO3-), carbonates (CO3--) and, in rare instances, hydroxide (OH-). Total alkalinity is the sum of the carbonate and bicarbonate alkalinities. Some waters may contain only bicarbonate alkalinity and no carbonate alkalinity.
The carbonate buffering system is important to the fish farmer regardless of the production method used. In pond production, where photosynthesis is the primary natural source of oxygen, carbonates and bicarbonates are storage area for surplus carbon dioxide. By storing carbon dioxide in the buffering system, it is never a limiting factor that could reduce photosynthesis, and in turn, reduce oxygen production. Also, by storing carbon dioxide, the buffering system prevents wide daily pH fluctuations.
Without a buffering system, free carbon dioxide will form large amounts of a weak acid (carbonic acid) that may potentially decrease the night-time pH level to 4.5. During peak periods of photosynthesis, most of the free carbon dioxide will be consumed by the phytoplankton and, as a result, drive the pH levels above 10. As discussed, fish grow within a narrow range of pH values and either of the above extremes will be lethal to them.
In recirculating systems where photosynthesis is practically non-existent, a good buffering capacity can prevent excessive buildups of carbon dioxide and lethal decreases in pH. It is recommended that the fish farmer maintain total alkalinity values of at least 20 ppm for catfish production. Higher alkalinities of at least 80-100 ppm are suggested for hybrid striped bass. For water supplies that have naturally low alkalinities, agriculture lime can be added to increase the buffering capacity of the water.
Hardness
Water hardness is similar to alkalinity but represents different measurements. Hardness is chiefly a measure of calcium and magnesium, but other ions such as aluminum, iron, manganese, strontium, zinc, and hydrogen ions are also included. When the hardness level is equal to the combined carbonate and bicarbonate alkalinity, it is referred to as carbonate hardness. Hardness values greater than the sum of the carbonate and bicarbonate alkalinity are referred to as non-carbonated hardness. Hardness values of at least 20 ppm should be maintained for optimum growth of aquatic organisms. Low- hardness levels can be increased with the addition of ground agriculture lime.
Other Metals and Gases
Other metals such as iron and sodium, and gases, such as hydrogen sulfide, may sometimes present special problems to the fish farmer. Most complications arising from these can be prevented by properly pre-treating the water prior to adding it to ponds or tanks. The range of treatments may be as simple as aeration, which removes hydrogen sulfide gas, to the expensive use of iron removal units. Normally iron will precipitate out of solution upon exposure to adequate concentrations of oxygen at a pH greater than 7.0.
Appendix 1
|
TABLE 3 Suggested water-quality criteria for aquaculture hatcheries or production facilities.
|
|
| Chemical | Upper Limits for Continuous Exposure and/or Tolerance Ranges |
|---|---|
| Ammonia (NH3) | 0.0125 ppm (un-ionized form) |
| Cadmiuma | 0.004 ppm (soft water < 100 ppm alkalinity) |
| Cadmiumb | 0.003 ppm (hard water > 100 ppm alkalinity) |
| Calcium | 4.0 to 160 ppm (10.0-160.00 ppm d ) |
| Carbon dioxide | 0.0 to 10 ppm (0.0-15.0 ppm d) |
| Cholorine | 0.03 ppm |
| Copperc | 0.006 in soft water |
| Hydrogen sulfide | 0.002 ppm (Larsen - 0.0 ppm) |
| Iron (total) | 0.0 to 0.15 ppm (0.0-0.5 ppm d) |
| Ferrous ion | 0.00 ppm |
| Ferric ion | 0.5 ppm (0.0-0.5 ppm d) |
| Lead | 0.03 ppm |
| Magnesium | (Needed for buffer system) |
| Manganese | 0.0 to 0.01 ppm |
| Mercury (organic of inorganic) | 0.002 ppm maximum, 0.00005 ppm average |
| Nitrate (NO-3) | 0.0 to 3.0 ppm |
| Nitrite (NO-2) | 0.1 ppm in soft water, 0.2 ppm in hard water |
| Nitrite-nitrogen | 0.03 and 0.06 ppm nitrite-nitrogen |
| Nitrogen | Maximum total gas pressure 110% of saturation |
| Oxygen | 5.0 ppm to saturation; 7.0 to saturation for eggs or broodstock |
| Ozone | 0.005 ppm |
| pH | 6.5 to 8.0 (6.6-9.0d) |
| Phosphorus | 0.01 to 3.0 ppm |
| Polychlorinated biphenyls (PCBs) | 0.002 |
| Total suspended and settleable solids | 80.0 ppm or less |
| Total Alkalinity (as CaCO3) | 10.0 to 400 ppm (50.0-4.00.0 ppmd) |
| % as phenolphthalein | 0.0 to 25 ppm (0.40 ppmd) |
| % as methyl orange | 75 to 100 ppm (60.0-100.0 ppmd) |
| % as ppm hydroxide | 0.0 ppm |
| % as ppm carbonate | 0.0 to 25 ppm (0.0-40.0 ppmd) |
| % as ppm bicarbonate | 75 to 100 ppm |
| Total Hardness (as CaCO3) | 10 to 400 ppm (50.0-400.0 ppmd) |
| Zinc | 0.03-0.05 ppm |
| Salmonid quality standards with modification for warmwater situations. Concentrations are in ppm (mg/1). (Source: Modification from Wedemeyer, 1977; Piper, etc al. (Larsen), 1982) | |
| a To protect salmonid eggs and fry. For non-salmonids 0.004 ppm is acceptable b To protect salmonid eggs and fry. For non-salmonids 0.03 ppm is acceptable c Copper at 0.005 ppm may suppress gill adensione triphosphatase and compromise smoltification in anadromous salmonids. d Warm water situations |
|
Further Reading
|
|
|
October 2007

