Sturgeon are raised primarily for their eggs to make caviar and for their high quality meat. The meat of both males and females is firm and white, and caviar is obtained from sexually mature females. In a typical sturgeon production cycle, depending on the species, males are harvested for meat first (at 2–4 years of age), and the females are retained for caviar production (until 5–9 years of age). Because management strategies for sturgeon depend on the sex of individual fish, being able to determine the sex of individual sturgeon has specific economical advantages.
The determination of sex in sturgeon is difficult because sturgeon do not possess easily distinguished external characteristics to differentiate females from males, especially when the fish are young (from hatch to 3 years old). Production of all-female populations through chromosome manipulation, a routine procedure for other fish species like salmonids, has as yet not been successful with sturgeon.
At present only a few techniques are available for the identification of sex in sturgeon. Measurement of reproductive hormone concentration (e.g., estrogen, progesterone, testosterone) or other metabolic indicators like the yolk precursor, vitellogenin, in the blood or mucus is possible, but the samples take at least a day to analyze in a laboratory, and currently no portable or field tests are available.
There are only two techniques that can immediately identify sex in sturgeon: 1) direct observation of the gonad using a surgical procedure, or (2) the use of an ultrasound machine. A method that is becoming more widely used is to scan the abdominal cavity of the fish using an ultrasound machine with an appropriate transducer and differentiate the gonad into an ovary or testis. This method has proven to be reliable, but primarily with gonads that are well differentiated, which are usually found in older individuals, at approximately 36 months in age. The procedure is not as accurate when used to discern the sex of early differentiated gonadal tissue, as in young individuals (12–24 months). In this paper we describe the standard method and tools for determining the sex of sturgeon by direct examination of the gonad using a minimally invasive surgical procedure.
The surgical procedure is performed through a small incision made in the abdominal cavity of each fish in order to look directly at the gonad, insert an instrument (e.g., endoscope, laparoscope, boroscope, etc.), or take a biopsy sample. The gonad or biopsy sample can be examined with the unaided eye (macroscopically), using a magnifying glass, or the tissue sample (the biopsy; either fresh tissue or tissue processed for histological analysis may be used) can be examined under a microscope. The greatest advantage of the surgical biopsy of the gonad is that it is the most reliable method to identify the sex in sturgeon, especially at an early age (16–36 months), and to determine the stage of sexual maturity of the gonads (the publications FA153 and FA154, explain the procedure for obtaining mature oocytes to determine their polarization index and thereby estimate maturity). The surgical procedure to obtain a biopsy of the gonad is the “standard” for comparison, to verify the accuracy of alternative methods that attempt to identify the sex in sturgeon. The technique is suitable for use in the field and is safe, with minimal complications. In thousands of surgeries we have performed since we developed the technique in the early 1980s, the incidence of reported mortality has been very low. When mortalities have occurred, they have usually been associated with infections, especially when the fish have been handled in high water temperatures (above 26°C) or in conditions of poor water quality. The procedure is performed within a short period of time, usually taking no more than two minutes per fish, and using relatively simple and inexpensive surgical tools.
The technique does have disadvantages: it is still invasive (although minimally); potential for infection is increased; and determining the sex requires up to a couple of minutes to perform on each fish. Also, if a surgical suture is used, the cost for the material for each fish may be around $1 USD. Finally, though the procedure is relatively simple, developing speed and skill requires some practice. Handlers with no experience of this method are strongly encouraged to consult with a fish veterinarian and an experienced aquaculturist before attempting the procedure.
Materials
- A source of clean, aerated fresh water.
- Anesthetic compound.
- A tank to contain the fish.
- Fish handling and holding equipment: bobcat or backhoe with a lift net, live cars, stretchers, and sawhorses.
- Surgical tools: surgical gloves, scalpel handle (e.g., no. 3) and blades (e.g., nos. 10, 11, 15). Tissue forceps: Adson-Brown (with 7x7 teeth) and Allis tissue forceps (4x5 teeth). A heavyweight needle holder/scissors (e.g., Olsen-Hegar) to hold the needle and cut the suture. Absorbable suture materials (regular or antibacterial) for tissue ligation: Vicryl (polyglactin 910) size 0 or 1 with OS-6 or 4 reverse-cutting needle; PDS II (polydioxanone) sutures, size 1 with CP-1 cutting needle.
- Miscellaneous: data sheets or notebook (preferably waterproof); pens/pencils; tackle box or similar container in which to store the surgical tools, sutures, etc.; small container to hold the surgery tools in disinfectant; hand and/or paper towels; small table on which to place the surgery tools, sutures, and other sexing materials; sterile wipes and alcohol or iodine for cleaning/disinfecting instruments in between fish; containers to serve as waste baskets and to contain “sharps” for the used blades and suture needles; a good, strong light source to allow for clear observation of the gonad.
Protocol
Minimally invasive surgery to determine sex in sturgeon is safe and effective. A skilled operator can perform some 300 to 500 surgeries in one day. Some elements of the procedure (i.e., method of anesthesia, and the decision whether to suture or not) are limited by government or animal welfare regulations, the comfort level of the operator, or the potential for infection. The overall goal of the surgical procedure in a commercial operation is to remove or separate males and to select females with obvious, easy-to-see ovaries. When the sex of the fish is difficult to determine, they are often classified as "unknowns" and may be separated or simply sold as meat fish.
Capture and Anesthesia
First, sturgeon are gently captured using nets or stretchers to minimized injury and stress. The fish should then be anesthetized until they roll ventral side up and venting of the gills slows down. Several drugs have been used for sedation and anesthesia of sturgeon. The only approved anesthetic for use in fish in the USA is MS-222 (Tricaine-S; FinquelTM), but unfortunately this is currently not approved for use in sturgeon. The drug requires treated fish to have a minimum of 21-day clearing (depuration) period before the fish can be used for food. MS-222 is known for its acidic nature due to the formation of methane-sulfonic acid, especially in soft, low alkalinity water. The pH of the water should be checked and if it is too low (less than 6.5) the anesthetic bath (e.g., 100 ppm) should be buffered with sodium bicarbonate. Two other drugs that are effective but that are not approved for use in the USA include 2-phenoxyethanol (e.g., 300 mg/L), and benzocaine (e.g., BZ-20 100–150 mg/L).
Until anesthetic drugs are approved for use on sturgeon, non-drug options are the only alternatives. The clove oil derivatives Aqui-STM 75-150 mg/L and Aqui-S20E (476-537 mg/L) have been used. Aqui-S is not legal for use in the USA; however, Aqui-S20E is currently allowed under an FDA Investigational New Animal Drug (INAD) exemption in hatcheries, with a withdrawal period of 3 days. Sturgeon have been successfully anesthetized by immersing them in chilled water (10–12°C) and administering CO2 gas combined with pure oxygen to the water. After capture and anesthesia, the fish is placed ventral (abdomen) side up in a stretcher. Experienced personnel usually will keep the fish out of the water no more than 2–3 minutes, but in case of unexpected mishap, keep handy a fresh, non-chlorinated water source to irrigate the gills. In addition, properly designed stretchers with water drain holes on the upper sides and sawhorses that are slightly lower in the front will create a reservoir of water that can be just deep enough to immerse the head and gill region of each fish and keep the abdominal region above water to permit the surgery.
Disinfection
The surgery should be conducted in as clean as possible, aseptic conditions. Wear surgical gloves and keep the surgical tools clean and disinfected. Minimal disinfection can be achieved by simply washing with detergents and soaking the instruments in solutions such as 70% ethyl alcohol, 65–90% isopropyl alcohol, or 1–3.5% iodine (note: iodine concentrations greater than 3.5% are toxic to tissue and do not provide additional disinfectant activity). Sturgeons secrete copious amounts of mucus that appear to have antibiotic properties, so the need for surface disinfectants is optional. Actually, we do not recommend swabbing of the incision area with a disinfectant because many surgical scrub solutions appear to irritate or even damage the fish’s skin and remove the naturally protective mucus coating. If there is a great deal of mucus, a gentle swab of the incision site with a sterile gauze pad moistened with a dab of 1% iodine solution is all that is needed.
Incision
The preferred surgical incision area is below the liver on the right side of the fish, where the right gonad may be relatively easily exposed and accessed; the gonad on the left side is less accessible because it is more covered by the spleen. The cut should be some 1–5 cm off the ventral mid-line, opposite 3–5 ventral scutes anterior from the pelvic fin (Figure 1). This should place the opening directly or almost directly above the gonad. The exact location for the incision will depend greatly on the species and the unique physical characteristics of each individual fish—sturgeon display considerable variation in body conformation and scute patterns. In most cases, the gonad should be immediately visible through the incision.
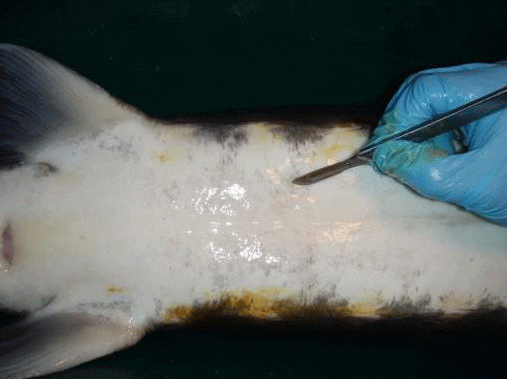
Use a scalpel and tissue forceps to make the cut. The incision should be 1–3 cm long; the length will depend on the specific method you use. You will need a shorter incision if you use an endoscope to see the gonad and a somewhat longer one if you use forceps to remove a sample of gonad tissue. A single through-cut, made without lifting the blade will best allow you to avoid tearing the skin, and will prevent excessive bleeding and encourage healing. The mid-line ventral skin in sturgeon is poor in blood supply, so make the cut slightly off-center through the relatively well-vascularized muscle tissue in order to enhance the healing process. Make a swift incision and make your examination of the gonad as rapid as possible to take advantage of the slight delay before the incision starts to bleed.
Examination
In some cases the gonad is very small or fatty. A pair of Allis tissue forceps (4x5 teeth) can be used to help manipulate and roll the gonad so that the ovary or testis tissue can be observed; if necessary the forceps also can be used to take the biopsy. You will need a bright head- or hat-mounted flashlight, shop lights, or some other type of lighting to clearly observe the gonad; some people are proficient at seeing the gonad simply in daylight by placing the fish at a proper angle.
The gonads in sturgeons are paired and consist both of fatty (adipose) tissue and the “germinal tissue,” where the actual eggs or sperm develop (Figures 2). The color of the gonad varies (white to yellow to orange) depending on husbandry conditions, such as diet. The greatest challenge is to differentiate the ovarian tissue from the adipose tissue. The female ovarian tissue is typically darker than the testis, usually an off-white to yellowish color. However, the distinguishing characteristics are the “ovigerous folds”—the compartments in which the eggs develop. These are shaped in folds so that they look somewhat like draped cloth. Seen from above, ovigerous folds resemble keys on a piano. The ovarian tissue also has a grainy appearance formed by the eggs developing within the folds (Figures 2b and 3a). Some fatty tissue is present in the male gonads, but the testicular tissue is smooth, turgid, and whitish in color; mature testes look like large, milky-white lobes (Figures 2b & 3b). Occasionally you will find a mature male. In these cases, if the testis tissue is broken, you may see the white and creamy-viscous testicular fluid.
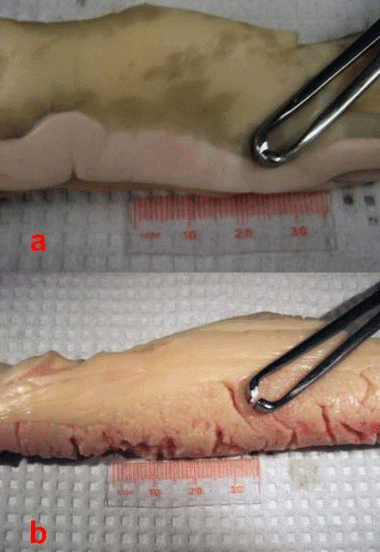
Because the gonad in sturgeon is open or exposed in the abdominal cavity, it can also be differentiated by palpating it with the fingers. In female fish, you will feel the grain of the eggs and the ovigerous folds (Figure 3A). The testis, in constrast, will feel smooth to the touch (Figure 3b).
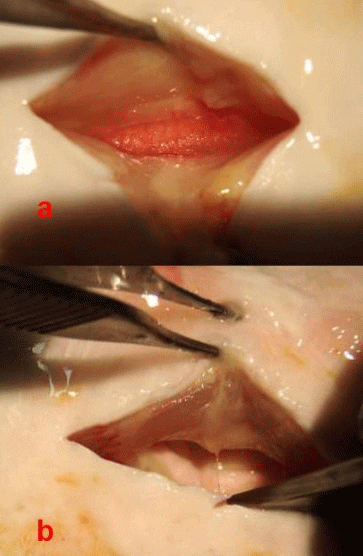
b. Male testis without fatty tissue; note the distinctly white, lobe-like subdivisions; surface will feel smooth to the touch.
If after looking and feeling you still cannot discern the sex of the fish, remove a small tissue sample with the Allis forceps, a biopsy punch or other suitable tool. Take the biopsy from the germinal tissue of the gonad and drop it into a preservative (e.g., 10 % neutral buffered formalin or a solution above 70 % alcohol) for further histological processing and examination of the tissue under a microscope. We have observed that if the tissue sample sinks in the preservative/holding solution, it is from a male. The tissue of the testis is denser than ovarian tissue because it contains cysts full of developing spermatozoa.
Suturing
Once you have determined the sex of the individual sturgeon, you may wish to close the incision with a suture material. In some commercial operations, when thousands of individual sturgeons potentially need to be sexed, the incision is often not sutured. If the incision is short (less than 2 cm), the healing process will be relatively quick and cause minimal tissue trauma. In thousands of surgical interventions we have performed, we have seen no mortalities caused by unsutured incisions. In fact, wounds appear to heal more quickly, we have seen no infections, and the fish recuperate faster than those undergoing anesthesia and suturing. In operations that use water recirculation systems or pond culture, however, suturing could help prevent healing complications and secondary infections due to water quality, environmental bacteria, or exposure to a mud bottom.
Suturing requires practice and a simple kit of proper tools (Figure 4). The surgery kit required includes a scalpel handle and blades, an Adson-Brown tissue forceps, and a heavy needle holder with serrated jaws, preferably combined with suture scissors. The Adson-Brown forceps with 7x7 side grasping teeth are ideal since they have small teeth that grip the slippery skin of sturgeon but at the same time reduce tearing the skin. A surgical needle holder with a pair of heavy grasping jaws (e.g., for wire), greatly facilitates holding of the needle, which must be of high caliber in order to pierce the sturgeon's tough skin. A combination needle holder/scissors will facilitate the suturing process by eliminating the need to replace the needle holder with a separate scissors. Absorbable sutures that are strong, pliable, and attached (swaged) to reverse-cutting needles are best for use in sturgeons (e.g., PDS II and Vicryl sutures of thread gauge size 0 or 1, attached to CP-1 and OS-4/6 needles, respectively or the more recent antibacterial versions of these materials). Swaged needles are secured to the end of the suture so that the diameters of needle and suture are nearly equal in size and nearly smooth. This type of suture causes minimal trauma to the sturgeon’s skin, which is tough, not very elastic, and often embedded with miniscule dermal scutes (Chapman & Park 2005). The use of plain surgical gut (chromic) suture has worked well on sturgeon but only when they are held in cold water (e.g., below 15 °C) until they heal completely. At warmer temperatures the surgical gut suture breaks before healing is completed (which will require some 20–30 days to heal completely), and the tissue becomes inflamed. The other types of materials for wound closure (tissue glue, staples, etc.) have yet to be tested thoroughly in sturgeon to determine if they work better than the traditional thread-sutures.
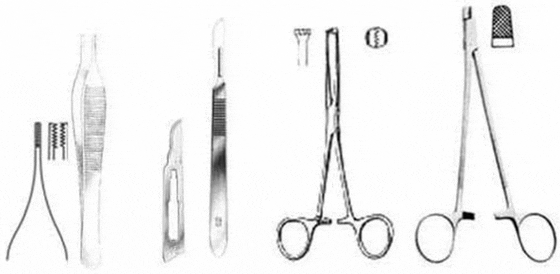
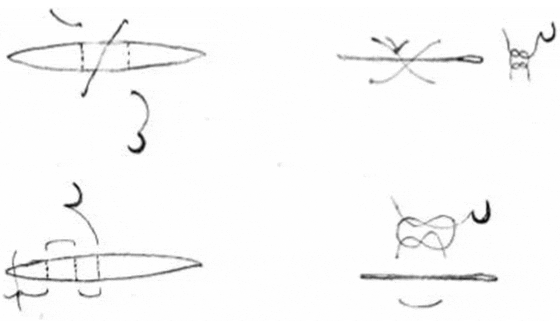
For suturing or stitching and tying knots, use basic surgical suturing techniques (e.g., Giddings 1997). A small cut (1–3 cm) can be sutured with an interrupted, X-suture pattern, tied with a surgeon's knot, and secured with a second and third knot (Figure 5). For a long incision (3–5 cm or longer), we recommend a horizontal mattress suture (Figure 5), primarily because it is strong and tends to turn the inner surfaces of the skin outward (eversion), which facilitates the healing process. A good suturing technique is needed to ensure fast healing.
July 2013


