Read it as a guide-line only as no advice on health management is ever absolute. The steps outlined should help farmers overcome a very serious problem with very simple techniques. The techniques in the guideline must be personalised to your own farm as treatment and dosage might vary according to fish, pathogen and environment. Eventually, these techniques should help improve husbandry and contribute to better profit.
In order to create a personalised Parasite Prevention Plan (PPP) two issues need to be clear;
-
A clear understanding of your own farming situation
-
A good knowledge of marine water parasites present in your farm.
As a reference, background information on the major important parasitic diseases of cultured marine fish in the Asia-Pacific region can be found in the Technical articles located under What’s new? section.
 |
| Small scale wooden (single/multi species) |
 |
| Large scale commercial PVC cages |
Below is a list of questions marine fish farmers should ask themselves to get a clear understanding of their farming situation:
-
Know your culture system. There are two main cage systems in the Asia-Pacific region; Small scale wooden (single or multi species) and large scale single species commercial PVC cages.
-
Know your environment. Are your cages exposed or sheltered and what is the proximity to other farms?
-
Know your husbandry techniques. What species do you have, what is the age and size, how long and how big will you grow them?
- Analyse your production records for any noticeable trends (or changes) in growth curves, daily mortalities and FCR’s. Fish performance and growth is affected by parasitism. This relationship can be observed by comparing performance records between different periods or seasons and by correlating them with parasite outbreaks.
Parasites can affect the farmed fish in two different ways: Chronic severe disease outbreaks and low grade consistent mortality. In both cases, the detrimental effect of parasites can manifest itself by reduction of growth, poor FCR, loss of appetite and even mortality. Moreover, once fish are infected, they will be more susceptible to concurrent bacterial or viral infections.
Once these farming parameters are known, it is important to know which marine water parasites are in the farm. This is the first step in parasite management:
Step 1: “On site” parasite observation
 |
| Neobenedenia sp. (skin fluke) on Asian sea bass |
 |
| Zeylanicobdella arugamensis sp. (leech) on Pompano |
To identify the parasites on your farm you will need some basic equipment. You will need a microscope, some histology glass slides and cover slips. Firstly, sacrifice a few ‘apparently’ sick and healthy fish for sampling. Using a cover slip, scrape the dorsal side of the fish and smear the mucus onto a slide. For both small and large fish cut out a section of the gills (a gill filament) place it on a slide with a drop of marine water and put the cover slip on top. In order to scan quickly for parasites under the microscope start with magnification x10. Then zoom down to x40 for more detailed species identification. In addition to a microscopic analysis, some parasites are visible with the naked eye. For example, skin flukes such as Neobenedinia sp can be seen by simply dipping the fish in a black freshwater bucket for five minutes: parasites will turn from clear to opaque and will be easily seen by observing the water in front of a black background. Other parasites such as isopods and leeches are big enough to be seen with the naked eye.
As a reference, pictures of major tilapia parasites can be found in Intervet’s AAH Newsletter 14. Species names of parasites in marine water fish might differ from the thoses found in tilapia, however, the shapes of parasites from similar families or genus will look alike independent of the salinity of the water.
Step 2: ‘Paragram’ (Parasite Prevention Programme-PPP)
 |
| Rhexanella sp. (Isopod) on Snapper |
Once the fish culture system is understood and the parasitic fauna of the farm is identified, it is time to create a personal PPP. To do so, we need to know which treatments are effective for which parasites. For marine parasites, there are various treatments available. These may include synthetic drugs, chemicals and freshwater. Antibiotics are not recommended as part of a regular PPP as they have a specific action towards bacteria and have no effect on parasites.
Before choosing a treatment, appreciate that there may be serious consequences from applying the wrong treatment for the wrong reasons. For example, potassium permanganate becomes toxic in salt water by creation of manganese complexes in fish gills. Similarly, some species are more susceptible to some treatments than others, such as the Humpback grouper (Altivelis cromiliptes sp), which are easily stressed by hydrogen peroxide. It must be emphasized that in most countries, only a few drugs and chemicals have been registered for treatment of food fish. It is wise to consult your local fish health expert before the plan starts as the treatment used must be safe for the fish, the environment and the consumer. It is important to state that any therapeutic compound used on food fish must be legally authorized in the country of use.
Effective treatments and useful suggestions are widely available in books on aquaculture or on the internet. However, do not blindly follow information on fish treatment. It is advised to try a new treatment on a small experimental scale and perform a ‘paragram’ as outlined inthis article. To do this, you will need some buckets, infected fish, a range of treatments and an oxygen supply. The oxygen supply can be aeration with an air pump or pure oxygen. In the case of a pure oxygen supply, careful monitoring of the dissolved oxygen present in the treatment water is necessary as super-saturation of the water may occur causing ‘gas embolism’ in the fish.
As a preliminary experiment, three types of treatment can be tested: Hydrogen peroxide which is recognised as a standard chemical in parasite management, formalin and freshwater. Collect some infected fish and split them equally amongst 9 buckets of equal water volume. In each of your buckets place a low, medium and high dose of each treatment. Monitor the presence of parasites on the fish before and after application. An effective treatment should help to eliminate the parasites on the fish immediately after treatment.
Case example:
In the following example, a small scale marine cage farmer has observed gill flukes and ciliated protozoan in his snapper and skin flukes in his sea bass.
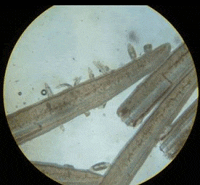 |
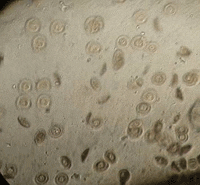 |
|
| Gill flukes in sea bass | Trichodina spp. in snapper |
After trying the three types of treatment mentioned above, fill up the following table that summarizes the presence or absence of parasites for different doses and treatments.
In general, the efficacy of a treatment is a function of both time and concentration. For instance, at a given concentration, a shorter exposure than what is required might not be effective to kill parasites. On the other hand, a longer application might be detrimental to the fish health, as it is stressful and might cause water quality deterioration. Consequently, the objective of the paragram is to find out the minimum concentration of each treatment required to eradicate each of the parasite species present on the fish.
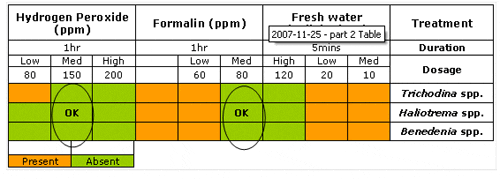
In this example, our farmer decides he would like to use hydrogen peroxide as a bath treatment for all of his fish at the dose of 150 ppm for 1 hour.
Parasites are part of the natural ecosystem and complete eradication is extremely unlikely. However, by repeating this treatment on a regular basis, the farmer designs his own parasite prevention plan as a way to control the parasite pressure on his fish before it gets dangerous.
Step 3: Step by step to treatment as part of the PPP
As previously mentioned, it is necessary to understand the parasite cycle in order for the treatment to be effective. In our example, fish are infected with capsalid monogeans that lay eggs on nets to complete their life cycle. It is therefore very important to include the nets in the treatment. The life cycle for this parasitic species is ten days so a preventative treatment needs to occur consecutively within that time frame. Frequency of treatment might vary depending on temperature, parasite and fish susceptibility to parasites. For instance, managing Cryptocaryon irritans, a protozoan parasite responsible for white spot disease, necessitates the application of at least four treatments applications in a row every three days. This is due to the existence of an encapsulated stage resistant to treatment in the life cycle of the parasite.
Currently, the most effective technique for managing parasites is to use a tarpaulin treatment as described below:
 |
| Tarpaulin enclosure of a small cage |
 |
| Treatment application including additional aeration |
-
Prior to treatment, or handling of any sort, starve the fish as feeding increases metabolic activity and causes undue stress.
-
With back up oxygen ready (either from a pure oxygen tanks or blowers), start to crowd the batch of fish for treatment. The maximal fish density applicable will vary from case to case as tolerance to stress varies from species to species.
-
Prepare the tarpaulin and start to pull it under the cage (ensure the entire net is included as well). Larger cages will need swimmers/divers to drag the tarpaulin underneath.
-
Open the air valves and (using a DO meter) continuously monitor the Oxygen and pH levels).
-
Apply the chemical as a splash treatment and monitor the fish closely for the entire treatment duration. Note that H2O2 has the added benefit of removing a certain amount of bio-fouling from nets and depending on the concentration, may temporarily increase DO levels.
-
If at any stage the fish display abnormal behaviour remove the tarpaulin. Signs may include: ‘gasping’ at the water surface, erratic swimming or ‘darting’, listlessness behaviour ‘hanging’ in the water column, separation of individuals from the shoal and or colour changes ‘turning black’.
-
After one hour remove the tarpaulin and monitor the fish performance. Wait until you are sure the fish are recovered from the treatment before feeding.
Once satisfied with the procedure in one cage, it is time to apply a plan for the whole farm. Although not always possible, as a global approach to managing parasites, it’s best to treat all your fish in one day as opposed to treating different batches day after day. This is simply because of the risk of re-infestation of treated cages from untreated neighbouring cages.
Step 4: Quarantine
This step should be applied for all future incoming batches of fish. Indeed, fingerlings sourced from different farms and/or countries may be excellent vectors for introducing new pathogens and particularly parasites. Therefore, to quarantine all incoming stock is an important step of the PPP. Quarantine involves a thorough inspection either with the local veterinary authority or a personalised on-site analysis (see step 1) and seclusion.
Locate the introduced batch of fish away from the rest of your stock in a different cage or, even better, a different farm site. Initially treat using a tarpaulin surrounding the cage before the fish can interact with the natural water body. Wait one week for the stress level to reduce and sample for parasites on a few specimens (step 1). When no parasites remain, it is time to re-introduce the stock in your farm. Once the quarantine stage is over, it is necessary to perform an on-site analysis every week on that batch to monitor the re-emergence of any parasites. Generally, smaller fish are more susceptible to physiological stress and attack by parasites than larger fish. Therefore, the most important checks are during the weeks and months after introduction onto the farm. Once fish reach 200-300 g, they become less susceptible to parasites.
Conclusion
Following this guideline should help Asian-Pacific marine fish farmers to keep parasite pressure under control from stocking to harvest. By doing so, they should be able to accurately compare previous production records to those since implementation and be one step closer to improve future production targets and profit margins.
Finally, it should be kept in mind that parasite management is only part of the overall health picture and and that other husbandry techniques are very important and must be conducted on a regular basis as well. For small scale marine fish farming, these measures include net changes, feed management, and strict hygiene standards. Finally, great emphasis should also been put on the management of bacterial and viral diseases.
December 2007




