A total of 216 Nile tilapia fish (Oreochromis niloticus) were randomly divided into four groups. Group 1 served as a control that was administered no radiation and no MRN-100 treatment. Group 2 was exposed only to γ-radiation (15 Gy). Groups 3 and 4 were pre-treated with MRN-100 at doses of either 1 ml/l or 3 ml/l in water for 1 week, and subsequently exposed to radiation while continuing to receive MRN-100 for 27 days.
The survival rate was measured, and biochemical and histopathological analyses of hematopoietic tissues were performed for the different treatment groups at 1 and 4 weeks post-radiation.
Exposure to radiation reduced the survival rate to 27.7%, while treatment with MRN-100 maintained the survival rate at 87.2%. In addition, fish exposed to γ-radiation for 1 week showed a significant decrease in the total number of white blood cells (WBCs) and red blood cells (RBCs) series.
However, treatment with MRN-100 protected the total WBC count and the RBCs series when compared with irradiated fish. Furthermore, significant histological lesions were observed in the hepatopancreas, spleen and gills of irradiated fish. However, treatment with MRN-100 protected the histopathology of various organs. We conclude that MRN-100 is a radioprotective agent in fish and may be useful as an adjuvant treatment to counteract the adverse side effects associated with radiation exposure.
Introduction
It is well known that ionizing radiation can cause a series of deleterious side-effects, including oxidative damage to cellular macromolecules and the demise of the hematopoietic system. Therefore, several investigators have directed their research towards finding effective radioprotective treatments that can successfully prevent or minimize the damaging effects of free radicals caused by ionizing radiation. Several agents have been shown to have some preventative properties; these include the synthetic agents amifostine and ethiofos, as well as several dietary supplements.
Although these products help alleviate the side effects of radiation, the synthetic compounds themselves have been shown to cause some toxicity. The radioprotective role of dietary agents including arabinoxylan rice bran (MGN-3/Biobran), genistein, bael leaf (Aegle marmelos), Panax ginseng, and eckol from marine alga were also investigated. Additionally, earlier studies indicated that antioxidants such as vitamin C and E can serve as effective radioprotectors. MRN-100, an iron-based compound derived from bivalent and trivalent ferrates (hydroferrate fluid), demonstrated its effectiveness as an antioxidant by preventing the formation of free radicals. Therefore, it is of special interest to examine its ability to act as a radioprotector against γ-radiation in the fish, Oreochromis niloticus.
Our work, and that of others, has shown that fish are excellent models for studying radiation effects on life span and hematopoietic tissues. Different fish species were used, including Oryzias latipes, zebrafish, Atlantic salmon, and Tilapia mossambica. Our recent study demonstrated that daily supplementation of aged rats with MRN-100 resulted in recovery of age-induced reactive oxygen species (ROS). Furthermore, MRN-100 provides a protective effect against H2O2-induced apoptosis of lymphocytes in vitro. Since the deleterious effects of ionizing radiation on living cells are often mediated by increased production of ROS, this study was undertaken to examine the radioprotective effects of MRN-100 against γ-radiation-induced lethality and damage to hematopoietic tissues.
Materials and Methods
MRN-100
MRN-100 was prepared in distilled water (DW) with the concentration of Fe2+ and Fe3+ ions at about 2 × 10-12 mol/l. MRN-100 is obtained from phytosin, a plant extract that contains iron and neutral lipid compounds and can be found in rice, wheat, or radish seeds. To start, 1 unit of phytosin is dispersed in 100 ml DW, and ferric chloride as FeCl3•6H2O is added. The lipid compounds are removed through a liquid–liquid extraction using a separation funnel. The remaining liquid is filtered with No. 5 filter paper and the filtrate is evaporated and condensed in a water bath. The iron compound obtained is subjected to fractional determination with respect to bivalent ferrate and trivalent ferrate in order to generate MRN-100. Briefly, the sample's Fe (II) quantity is determined using the o-phenanthrolin method. Hydroxylamine-HCl 10% solution is added to the sample liquid to reduce Fe (III) to Fe (II) beforehand. Subsequently, all of the ferrate quantities are determined, followed by the determination of the quantity of Fe (III). As a result, the iron compounds thus obtained turned out to be bivalent and tervalent ferrates. MRN-100 was provided by ACM Co., Ltd, Japan.
Radiation Schedule
Radiation was carried out with a γ-ray source of 4-MCi 137 Cs (Radiotherapy Department, Mansoura University Hospital, Egypt). The radiation employed for the wholebody radiation was a single dose of 15 Gy with a target object distance of 27 cm and an exposure rate of 200 R/min. During radiation, each group was kept in small oblong-shaped glass vessels containing 2 l of aged tap water. Immediately after radiation, fish were transferred into large, 200-l tanks, and 10% of the water was changed every day until the end of the experiment. Survival of fish was monitored twice-daily at morning and at night for 27 days post-radiation.
Oreochromis Niloticus
The Oreochromis niloticus (6–8 weeks old, ~50 ± 15 g body weight, length ~16.5 ± 10 cm) were purchased from a fish farm specializing in Tilapia (Iman Farm, Kafr El-Sheikh, Egypt) and acclimatized for 1 week prior to experimentation. The fish were placed in tanks (54 fish per tank); each tank contained 200 l of aged dechlorinated tap water. The number of fish per liter used in the current study was within the proximity of another published study. The fish were kept outdoors throughout the experimental period where the water in all aquaria was aerated continuously and temperatures were kept at ~ 22 ± 2ºC. The fish were fed standard laboratory floating pellets twice a day (at 8 AM and 1 PM). The water parameters remained constant throughout the experiment at dissolved oxygen (6–8 mg/l), pH (7.3–8.6), CO2 (10 mg/l), NH3 (0.02 mg/l), alkalinity (150 mg/l), hardness (180 mg/l), NO2 (0.01 mg/l) and NO3 (0.4 mg/l).
Protocol of the Experiment
A total of 216 Oreochromis niloticus were randomly divided into four groups (G1–G4) of 54 fish per group. Of these, 47 fish from each group were used for daily recording of survival post-exposure to radiation. (After 1 week, 5–7 fish of each group were used for hematology and histology studies. At 4 weeks, on the last day of the survival study, 5–7 fish of each group were used for follow-up hematology studies). G1 served as the control with no administration of MRN-100 or radiation; G2 was exposed to whole-body γ-radiation only; G3 and G4 were treated with doses of 1 ml and 3 ml of MRN-100 per liter of water, respectively, for 1 week, then exposed to radiation, while continuing to receive MRN-100 for 27 days. The effect of radiation and MRN-100 on the survival rate of fish was examined by recording the dead fish among the four groups daily for 27 days, while biochemical and histopathological analysis was performed on the surviving fish at 1 and 4 weeks post-exposure to γ-radiation.
Sample Collection
For biochemical analysis, 5–7 fish from each group were collected at 1 and 4 weeks post-irradiation and were treated with a mixture of clove oil and alcohol for 2–3 min. Each fish was put on a clean towel and 1–2 ml of blood was withdrawn from the caudal vein using a syringe containing EDTA. The tubes were thoroughly shaken to avoid clotting of blood and used for blood analysis, which include: white blood cell count (WBC), red blood cell count (RBC), hemoglobin content (HGB), hematocrit percentage (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelet count (PLT). Blood analysis was evaluated according to the protocol provided by the kit's manufacturer. In addition, 1 ml of blood from each fish was left to coagulate, then centrifuged and the sera were collected to evaluate the level of serum glutamic pyruvic transaminase (SGPT) in accordance with the protocol provided by the kit's manufacturer.
Histopathological Analysis
A range of organs, including the hepatopancreas, spleen and gills of each group, were examined for histopathological changes at 1 week post-exposure to γ-irradiation. Organs were fixed in 10% formalin solution, sectioned, and fixed overnight in cassettes. The paraffin-embedded tissues were sectioned on a microtome to a thickness of 4 μm, stained with hematoxylin and eosin (H&E) and observed under light microscopy.
Statistical Analysis
Values were reported as mean ± sd (standard deviation) for 5–7 fish in each group, and the significance of the differences between mean values of the fish under different treatment conditions was determined by one-way analysis of variance (ANOVA) coupled with the Newman-Keuls multiple comparison test. Values of p < 0.05 were considered to be significant.
Result
Several parameters were examined in irradiated fish in the presence and absence of MRN-100. These included survival rate, hematology, biochemical factors and histopathology.
Survival Rate
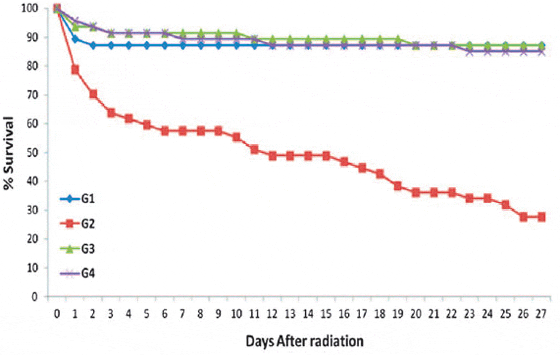
The effect of MRN-100 on the survival rate of γ-radiation is shown in Fig. 1. Exposure to γ-radiation resulted in death in 72.3% of G2 within 27 days. On the other hand, pretreatment of fish with MRN-100 (1 ml/ l and 3 ml/ l water in G3 and G4, respectively) caused remarkable improvement in fish survival, and only 12.8% of these fish were dead at the end of the experimental period.
There were significant differences in survival from Day 4 on between the γ-radiation group and the γ-radiation +MRN-100 groups (P < 0.02). There was no significant difference between the control (G1), and groups receiving MRN-100 (G3 and G4).
Fig. 1. Effect of MRN-100 on survival percentage in Oreochromis niloticus after ?-radiation. Fish were divided into four equal groups (G1–G4), each consisting of 47 fish. The dead fish among the groups were recorded daily for 27 days after exposure to radiation.
Hematology Examination
Several hematology parameters were examined in fish at 1 and 4 weeks post-exposure to γ-radiation in the presence and absence of MRN-100.
WBC Counts
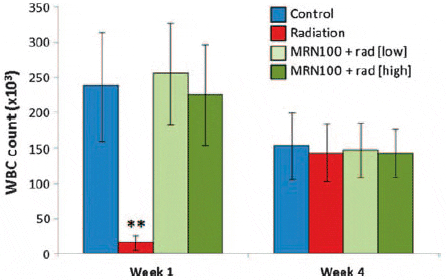
At 1 week, Fig. 2 shows that fish exposed to radiation only (G2) showed a highly significant decrease (93%) in their WBC counts; 16 × 103/mm3 as compared with 238 × 103/mm3 for control fish G1 (P < 0.01). However, pretreatment with MRN-100 in groups G3 and G4 resulted in maintenance of WBC counts of 256 × 103/mm3 and 223 × 103/mm3, respectively, and the changes were not significant compared with G1. At 4 weeks, the WBC counts of the four groups did not differ significantly.
RBCs Series
Effects of radiation and MRN-100 treatment on RBC series were examined at 1 and 4 weeks post-exposure to radiation. The parameters under investigation included RBC counts, HGB, HCT, MCV, MCH and MCHC. At 1 week, Fig. 3 shows that fish in the radiated group only (G2) demonstrated a significant decrease in their RBC counts, HGB content, HCT value, MCH content and MCHC compared with control values (P < 0.01 and P < 0.05). On the other hand, treatment with MRN-100 prior to radiation (G3 and G4) resulted in protection of the above-mentioned parameters, which then came within the levels of control untreated fish (G1). The statistical analysis comparing G2 with G3 or G2 with G4 showed significant change in all parameters with the exception of MCV. At 4 weeks, these values were not significantly different between the four groups.
The RBC and HCT values in the current study were low due to experimental conditions; all experiments were performed outdoors during the winter months. The seasonal influence on hematological parameters in fish is wellestablished. Several studies have shown that RBCs and HCT levels in different types of fish are significantly lower in winter than in the summer. These include Nile tilapia (O. niloticus), sea bream (Sparus aurata), rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax). The causal factors for these phenomena are attributed to the higher solubility of oxygen in colder water and to the reduced activity and reduced metabolism of fish in winter. Other environmental factors in addition to temperature should also be considered as contributory factors for the seasonal influence on hematological parameters in fish, such as photoperiod, water chemistry and spawning activity.
Fig. 2. Effect of MRN-100 on the WBC count of Oreochromis niloticus after ?-radiation. WBCs were isolated from the caudal vein and counted at 1 and 4 weeks post-radiation. Data represents the mean ± SD of 5–7 fish in each group compared with the control, untreated group. **P < 0.01.
Platelet Count
At 1 week, fish exposed to γ-radiation revealed no significant changes in the platelet count. Similarly, treatment with MRN-100 caused no changes in platelet count compared with the control group. Similar results were observed at 4 weeks (Fig. 4).
Biochemical Analysis (liver function)
Figure 5 depicts data on the effects of γ-radiation in the presence and absence of MRN-100 on the serum SGPT levels that were examined at 1 and 4 weeks post-radiation. Fish exposed to γ-radiation (G2) at 1 week revealed a 3.2-fold increase in the SGPT level (397.8 U/l) compared with the control (171.3 U/l) (P < 0.001). Pretreatment with MRN-100 resulted in decreasing the levels of SGPT in a dose-dependent manner; 210.0 U/ l at dose 1 ml/l, further decreased to 176.0 U/l at dose of 3 ml/l. At 4 weeks, no significant differences were observed among the four groups.
Histopathological Analysis
Histopathologic analysis was performed in different tissues in fish at 1 week post-exposure to γ-radiation in the presence and absence of MRN-100.
Hepatopancreas
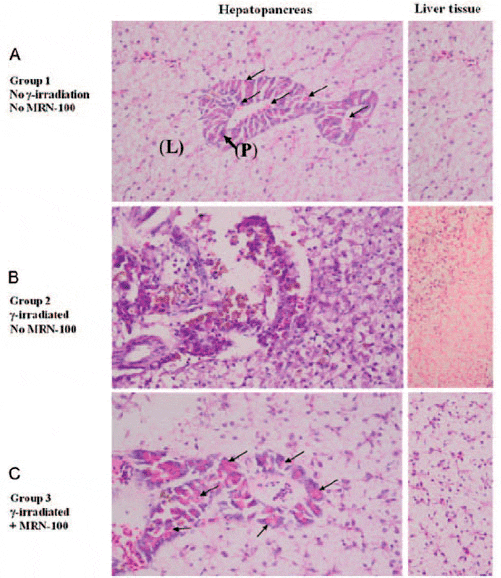
Figure 6 shows a pancreatic tissue inside the liver portion from control fish, and the presence of zymogen granules. Exposure to radiation caused dramatic changes in the hepatopancreas. Several histopathological lesions were observed including pancreatic necrosis, severely degenerative hepatocytes and loss of zymogen granules. When the fish were pre-treated with MRN-100 (Group 3) none of the histopathological lesions were observed and the hepatopancreatic tissue was similar to the control fish.
Spleen
The spleen of fish exposed to γ-irradiation revealed the absence of melanin pigments, hyperplasia in the melanomacrophage cells, and an increased amount of vacuolation (Fig. 7). When fish were treated with MRN-100 prior to exposure to radiation (G3), none of the histopathological lesions were observed in the splenic tissues and the spleens appeared similar to the control group, showing an increase in melanin.
Fig. 3. Effects of MRN-100 on in the RBC series of Oreochromis niloticus after ?-radiation and MRN-100 treatment. The RBC series include: RBC counts, HGB, HCT, MCV, MCH and MCHC. These parameters were examined at 1 and 4 weeks post-radiation. Data represent the mean ± SD of 5–7 fish in each group compared with the control, untreated group. *P < 0.01, **P < 0.05.
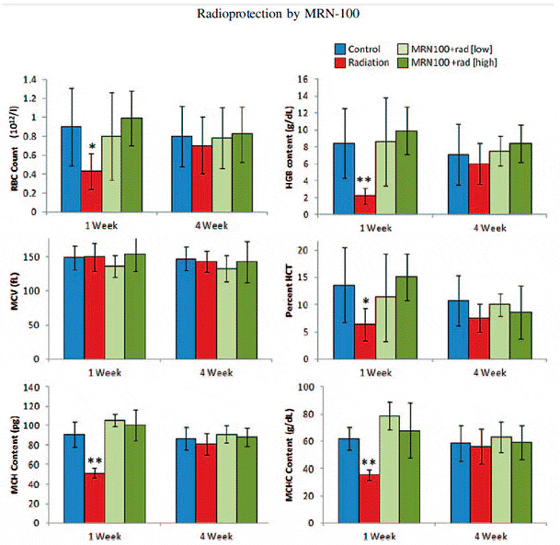
Fig. 4. Effect of MRN-100 on the platelet count (PLT) of Oreochromis niloticus after ?-radiation and MRN-100 treatment. Platelet counts were performed at 1 and 4 weeks post-radiation. Data represent the mean ± SD of 5–7 fish in each group compared with the control, untreated group.
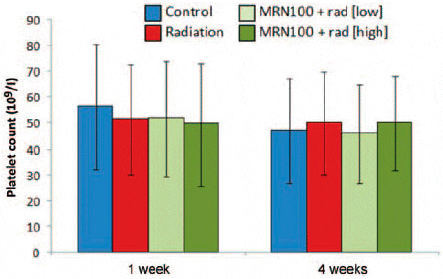
Fig. 5. Effect of MRN-100 on the SGPT level of Oreochromis niloticus after ?-radiation. SGPT levels were examined at 1 and 4 weeks post-radiation. Data represent the mean ± SD of 5–7 fish in each group. G2 was statistically significant compared with the control, untreated group. **P < 0.001.
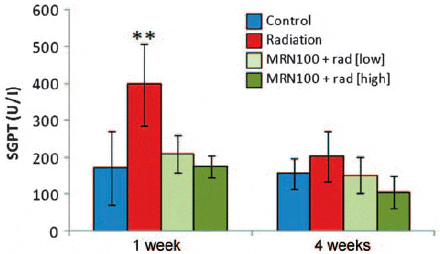
Fig. 6. Histological section of Oreochromis niloticus hepatopancreas at 1 week post-radiation. (A) Control hepatopancreas with normal histology, where the liver (L) is anatomically joined with the pancreatic tissue (P). Notice the presence of zymogen granules (arrows). (B) Irradiated hepatopancreas shows increased pancreatic necrosis and an increased number of severely degenerative hepatocytes in the liver portion. (C) MRN-100-treated and irradiated hepatopancreas. Notice the normality of the pancreatic and liver tissues and the presence of zymogen granules (arrows). (×40 magnification with H&E stain).
Gills
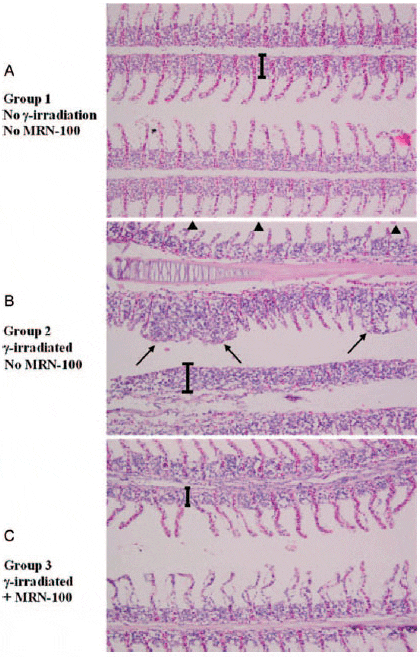
The gills are composed of a series of arch-like structures, from which radiate the gill filaments (primary lamellae). Figure 8A shows two gill filaments from control fish; projecting on their lateral sides are the secondary lamellae. Notice the gill filaments are covered by stratified squamous epithelial tissue. The gills of fish exposed to γ-radiation alone revealed several histopathological lesions, including an increase in thickness of the supporting cartilaginous rods and hyperplasia of the epithelial tissue (Fig. 8B). In addition, the secondary lamellae became shorter or disappeared completely from the surface of gill filaments, and lamellar fusion forming lumps were also noted.
When fish were treated with MRN-100 prior to exposure to radiation (G3), none of the histopathological lesions were observed in the gills or the gill filaments. In addition, secondary lamellae appeared normal, and the width of the epithelia tissue was similar to the control (Fig. 8C).
Discussion
Results of the current study revealed that MRN-100 could be used as a potential radioprotective agent, as exemplified by its ability to protect against γ-radiation lethality and damage to hematopoietic tissues. Exposure of animals to ionizing radiation causes a series of physiological changes known as acute radiation syndrome, which may lead to death with doses > 1 Gy due to severe damage to the hematopoietic system. Ionizing radiation is known to induce oxidative stress through the generation of ROS, resulting in an imbalance of the pro-oxidant and antioxidant status in the cells. ROS, such as •O2 - H2O2, •OH and •NO2, play a critical role in cell damage by inducing lipid peroxidation, protein modification, DNA damage, and cell death. The mechanisms by which MRN-100 protect against radiation-induced mortality in fish are not fully understood but could be attributed to the ability of MRN-100 to act as: (i) a potent antioxidant agent, and (ii) a protector of the gills from radiation damage. Regarding the antioxidant property, our earlier study showed that treatment with MRN-100 resulted in the recovery of age-induced ROS in rats. MRN-100 has proven effectiveness at increasing glutathione (GSH) concentration and antioxidant enzymes, accompanied by reduction in lipid peroxidation and free radical levels in the blood.
Fig. 7. Histological section of Oreochromis niloticus spleens at 1 week post-radiation. (A) Control spleen with normal histology with the presence of melanin pigments (white arrowheads). (B) Irradiated spleen. Notice the absence of melanin pigments, the hyperplasia in the melanomacrophage cells (yellow circles highlight), and the increased amount of vacuolation (yellow arrows). (C) MRN-100 treated and irradiated spleen. Notice the presence of dark melanin cells (white arrowheads). (×40 magnification with H&E stain).
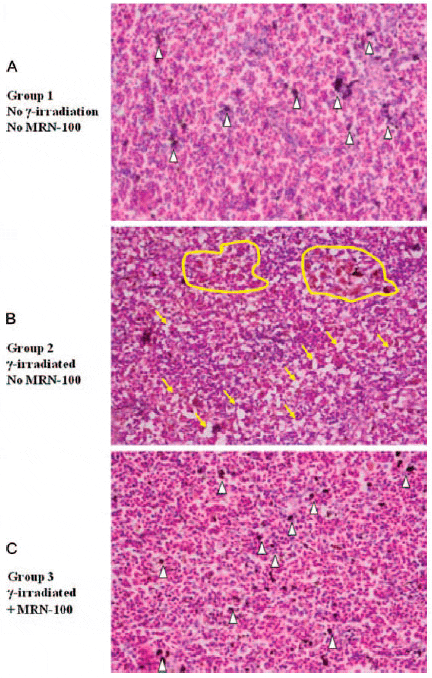
Fig. 8. Longitudinal sections of Oreochromis niloticus gills at 1 week post-radiation. (A) Control gill filaments and lamellae. The width of the epithelial tissue is indicated by a bracket. (B) Irradiated gill filaments. Notice an increase in thickness of the supporting cartilaginous rods. The hyperplasia of the epithelial tissue is indicated by the bracket. The secondary lamellae became shorter (arrowheads) or disappeared completely from the surface of the gill filaments, as seen in the gill filament on the bottom. Notice also the lamellar fusion forming lumps (arrows). (C) MRN-100 treated and irradiated gill filaments appear normal, with absence of the hyperplasia in the epithelial tissue. (×40 magnification with H&E stain).
This finding is in agreement with research into other antioxidants, such as vitamins C and E, which have been associated with an increase in the survival of irradiated mice. In addition, the increased mortality among γ-irradiated fish could be attributed to the significant damage to the gills, which include great destruction of the gill filaments and secondary lamellae, thus significantly reducing the oxygen supply to the body. On the other hand, treatment with MRN-100 prior to irradiation resulted in the absence of histopathological lesions in the gills, gill filaments and secondary lamellae. This indicates that treatment with MRN-100 provides protection for the gills from radiation damage.
The damage to the hematopoietic system is a major factor in mortality following acute radiation exposure. Our studies and those of others revealed atrophy of the hematopoietic tissues, a decrease in WBC counts, and immune dysfunction in fish and mammals during the first few days after radiation. In this study, fish exposed to γ-radiation for 1 week showed a significant decrease in the total number of WBCs. The mechanisms by which MRN-100 exerts its protective effect could be attributed to its ability to alleviate oxidative stress in lymphocytes. MRN-100 inhibits the apoptotic H2O2-induced signaling pathways by suppressing the H2O2-induced down-regulation of the anti-apoptotic molecule, Bcl-2, and upregulating the pro-apoptotic molecule, Bax.
Furthermore, oral administration of MRN-100 has resulted in a significant enhancement of natural killer (NK) cell activity. These innate immune cells are capable of destroying viral-infected cells and cancer cells. This characteristic suggests that the use of MRN-100 may result in protection against radiation-induced immune dysfunction. In addition, MRN-100 protected the fish from radiation-induced destruction of melanin pigments in the spleen. This is of particular interest because these pigments may be involved in defense mechanisms against disease and tissue damage. The results of the current study demonstrate that treatment with MRN-100 provides protection for WBCs and melanin pigments.
Anemia is often seen in patients with acute radiation syndrome, which results in decreases in erythrocyte counts and RBC parameters. Our data shows that exposure to radiation caused significant reduction in the RBC counts, HGB, HCT, MCH and MCHC, as well as formation of histopathological lesions in the splenic tissues. However, fish treated with MRN-100 prior to irradiation demonstrated protection against radiation-induced damage to the parameters of the RBC series, as well as prevention of histopathological lesions in the splenic tissues. Although the mechanism underlying the effect of MRN-100 is not fully understood, it is thought to regulate the levels of free iron in the cells.
Iron has the capacity to accept and donate electrons readily, thus it is the key catalytic site of many oxygentransporting proteins and enzymes. This characteristic makes it a useful component of cytochromes and oxygenbinding molecules. Nutritional deficiencies, such as lack of iron, have been shown to induce oxidative stress, and the ability of iron to protect against oxidative stress has been reported.
Iron is known to interact with free radicals in the Haber-Weiss and Fenton reactions, producing highly reactive hydroxyl radicals and carbon-centered radicals. MRN-100 may exert its effect by preventing excess iron from taking part in the Fenton reaction by stimulating increased protein levels of the iron-binding compounds ferritin and transferrin. Iron captured within ferritin, a key cellular iron storage protein, does not generate the reactive radicals that it might otherwise form.
Similarly, transferrin is responsible for sequestering and transporting iron through the bloodstream. It has been shown that the increase in transferrin and ferritin protein levels after MRN-100 treatment reduces the levels of free iron, and therefore prevents the accumulation of reactive radicals, such as protein carbonyl groups, a principal product of metal-catalyzed oxidation of proteins. Additionally, it is known that radiation-treated rats release free radicals.
Although there have never been any direct studies on the protective effect of MRN-100 in irradiated mammals, our recent study showed that MRN-100 has an antioxidant effect in aged rats. This would suggest that in irradiated rats, MRN-100 could have a protective effect by scavenging free-radicals, which may explain the ability of MRN-100 to induce the protection of RBC series and splenic tissues in irradiated fish.
In fish, the liver is anatomically joined with the pancreas, forming a single organ called the hepatopancreas. The hepatopancreas is the main target tissue of research related to radiation and pollutants that induce oxidative stress in fish. The oxidative stress induced by exposure to chemical pollutants has been well documented in different types of fish. Several classes of pollutants, such as copper and 1-methyl-3-octylimidazolium bromide, lead to the formation of ROS in the hepatopancreas.
Upon exposure to radiation, damage to hematopoetic tissue occurs due to a similar increase in the production of ROS. Therefore, we thought it of particular interest to examine the damaging effect of radiation on the hepatopancreas. The results of this study show that exposure to radiation causes dramatic changes in the hepatopancreas, including pancreatic necrosis, severely degenerative hepatocytes, and loss of zymogen granules. In addition, elevated levels of SGPT were observed in the irradiated fish, which is in accordance with other studies that show irradiation causes increased levels of liver enzymes.
However, treatment with MRN-100 prior to radiation significantly reduced the levels of SGPT, and none of the histopathological lesions were observed in the hepatopancreatic tissue. Similarly, treatment of MRN-100 in aged rats showed the liver, which has more oxidative damage than younger rat livers, had a significant increase in GSH, total thiol contents and antioxidant enzymes, in addition to a significant inhibition in lipid peroxidation, biomarker malondialdehyde, nitric oxide, and protein carbonyl groups. These findings may explain the protective effect of MRN-100 against radiation-induced damage to hematopoetic tissues.
The safety of radioprotectors is a major concern. The biosafety of MRN-100 has been studied in rats after 40 days of supplementation with no observable changes in animal behavior, body weight, or survival. Additionally, earlier studies have shown that healthy subjects and cancer patients who were administered MRN-100 orally for up to 12 months showed enhancement of immune function, such as NK cell activity.
We conclude that MRN-100 acts as a radioprotector by providing protection against radiation-induced mortality and radiation-induced hematopoietic damage in fish. This study suggests MRN-100 is a safe product that may be a useful adjuvant to counteract the severe adverse side effects that are associated with radiation therapy.
July 2013


