Introduction
Most monogeneans are browsers that move about freely on the fishs body surface feeding on mucus and epithelial cells of the skin and gills; however, a few adult monogeneans will remain permanently attached to a single site on the host. Some monogenean species invade the rectal cavity, ureter, body cavity, and even the blood vascular system. Between 4,000 and 5,000 species of monogeneans have been described. They are found on fishes in fresh and salt water and in a wide range of water temperatures.
Morbidity and mortality epidemics caused by excessive parasite loads are not uncommon in captive fishes and have also occurred in wild fishes. Captive fishes are usually held in more crowded conditions than fishes in the natural environment. This practice allows hatching monogeneans to easily find host fish. In addition, stressors found in the captive environment such as aggressive behavior by tankmates, poor nutrition, and poor water quality may impact the ability of the fishs immune system to respond to the presence of the parasites. Although monogeneans are commonly found on wild fish, they seldom cause disease or death in free-ranging populations because under natural conditions they are usually not present in high numbers on individual fish. However, any change that results in crowding of wild fish, which could include drought and diversion of water, may increase the density of parasites on wild fish and consequently result in disease. In addition, the release of monogenean-infested fishes to the natural environment can have potentially devastating effects. One example is the movement of resistant Atlantic salmon Salmo salar from Sweden that is suspected to be the source of Gyrodactylus salaris that caused heavy losses of susceptible salmon in Norwegian rivers. Another example is the introduction of monogenean-infested stellate sturgeon Acipenser stellatus from the Caspian Sea into Lake Aral that decimated the ship sturgeon Acipenser nudiventris population, which was not resistant to the monogenean.
Classification and Identification of Monogeneans
Though the terms monogenetic trematodes and flukes are often used to describe this group of parasites, both are incorrect because monogeneans are not trematodes or flukes. In fact, they are distinct from the other parasitic flatworms, which include turbellarians, tapeworms, and trematodes (the true flukes). Trematodes and tapeworms with rare exceptions only live internally in their host, and turbellarians occasionally parasitize the skin of marine fishes. At their posterior end monogeneans have a haptor, a specialized holdfast organ that has hooks or clamps that enable them to attach to their host (Table 1). Turbellarians, tapeworms, and trematodes do not have a haptor. The life cycle of monogeneans also differs from the life cycle of tapeworms and trematodes. Monogeneans have a direct life cycle, which means they go directly from host to host (fish to fish). Tapeworms and trematodes have an indirect life cycle that often requires multiple hosts (different types of animals).
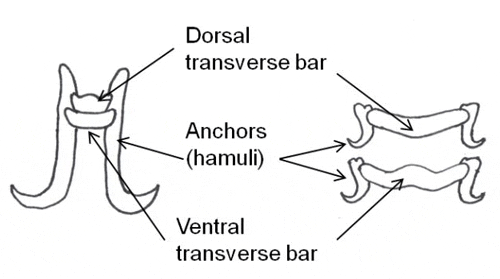
Monogeneans can be divided into two major groups, the monopisthocotyleans, which have hook-like organs on their haptors to attach to their host, and the polyopisthocotyleans, which use clamp-like structures for attachment. The polyopisthocotyleans are not as commonly diagnosed as the monopisthocotyleans, so they will not be covered in this article. The haptor is usually shaped like a disc. One to three pairs of hook-like structures called anchors or hamuli are commonly located in the center of the haptor. In many monogenean species, the anchors are used to attach to the host by penetration of the hosts skin. Anchors are often supported by ventral bars, transverse bars or accessory sclerites, which provide stabilization and/or attachment. Very small hooklets called marginal hooks are located near or on the periphery of the haptor and are the primary method of attachment for some monogeneans. Suction created using the haptor itself is another way some monogeneans stick to their host. Some species have adhesive glands on the haptor, and also on the head.
There are 13 families of monogeneans; of these, four are frequently diagnosed on aquacultured fishes. These four families are Gyrodactylidae, Dactylogyridae, Ancyrocephalidae, and Capsalidae (Figure 1), and parasites of these families are commonly called gyrodactylids, dactylogyrids, ancyrocephalids, and capsalids, respectively. Distinguishing characteristics include the presence or absence of eye spots, and the number of pairs of anchors (hamuli), transverse bars, and marginal hooks on their haptors. These features can only be seen with a compound microscope and are summarized in Table 2 and diagramed in Figure 2. The most common groups of monogeneans on marine fishes are the capsalids and ancyrocephalids. The most common groups of monogeneans on freshwater fishes are the gyrodactylids and the ancyrocephalids, which differ markedly in their reproductive strategies as well as their preferred attachment sites on host fish.
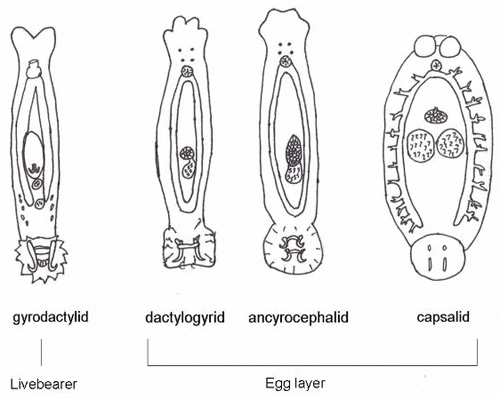
The majority of gyrodactylids are viviparous (produce live young) and are generally found on the bodies and fins of fish, though they occasionally may occur on the gills. Adult parasites carry fully developed embryos (identical to the adult), which in turn carry the young of the next generation. Each individual parasite represents several generations, which is why they are often referred to as Russian nesting dolls. This reproductive strategy allows populations of gyrodactylids to multiply very quickly, especially in a closed system. The few gyrodactylids that are egg-layers are usually found on members of the South American catfish families, Loricariidae (armored catfishes such as the pleco) and Pimelodidae (long-whiskered catfishes such as pictus cats). These gyrodactylids use an adhesive material to glue their eggs to the skin of the catfish. Gyrodactylids may be found on freshwater, marine, and brackish water fishes.
Gyrodactylids have a pair of anchors with both dorsal and ventral bars and 16 marginal hooks, and do not have eye spots. Attachment to the fish is made with the marginal hooks; the anchors are used as a spring-like device to assist attachment with the marginal hooks. An embryo with its pair of anchors may frequently be seen inside an adult gyrodactylid (Figure 3).
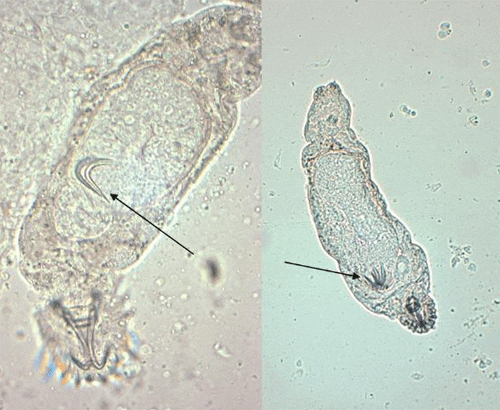
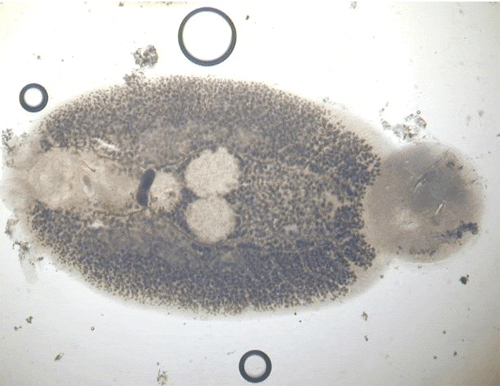
Dactylogyrids usually prefer the gills as a feeding and attachment site (Figure 4) and are primarily found on freshwater fishes belonging to the cyprinid family (danios, rasboras, barbs, goldfish, koi, freshwater sharks, etc.). An exception is Acolpenteron ureteroecetes, which may be found in the posterior kidney, ureters, and urinary bladder of infected largemouth bass Micropterus salmoides.
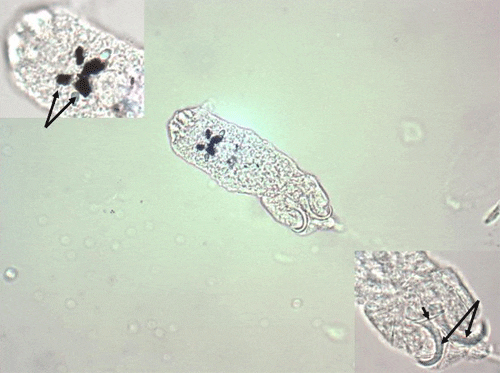
Dactylogyrids have two pairs of eye spots and occasionally have a vestigial pair of anchors in addition to a pair of anchors and one transverse bar. They usually have 1214 marginal hooks, but these may be absent in some species. Dactylogyrids are oviparous (egg-layers), and eggs are occasionally seen in association with gill tissue during microscopic examination.
Ancyrocephalids are closely related to the dactylogyrids. Unlike the dactylogyrids, they are not as selective about the host species of fish. They are usually found on gills of freshwater, marine, and brackish fishes. However, some genera (Neodiplectanotrema and Paradiplectanotrema) were reported in the esophagus of marine fishes, and one genus, Enterogyrus, lives in the stomach of African and Asian cichlid species.
Ancyrocephalids have two pairs of eye spots, and two pairs of anchors (Figure 5). Each pair of anchors has a transverse bar. Marginal hooks usually number 1214, but may be absent in some species. Some species produce an adhesive substance to assist in attachment to the fish host. Ancyrocephalids are oviparous, and eggs are occasionally seen in mucus when examining gill tissue with a microscope.
Capsalids (Figure 6) are primarily found on the skin and occasionally the eyes of marine fishes, and occasionally on brackish fishes. Some genera of capsalid monogeneans, Neobenedenia and Benedenia, for example, can cause chronic problems in marine aquaria and can be difficult to eliminate from aquaria once they become established.
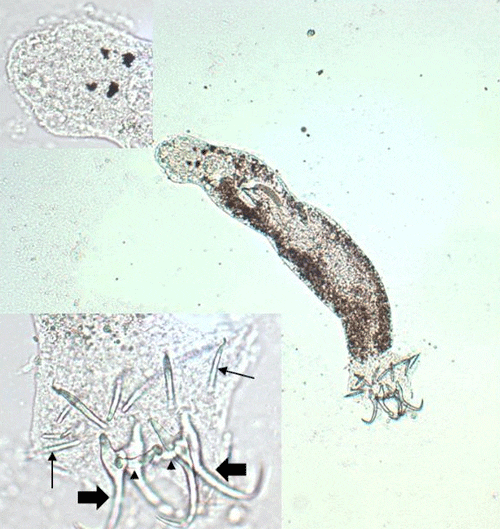
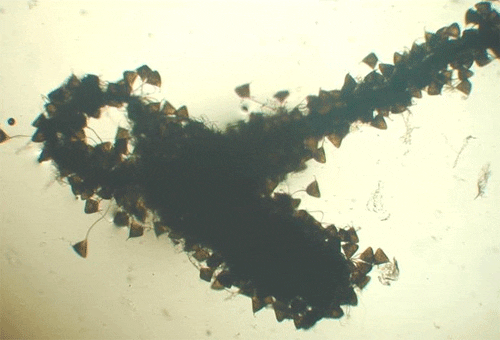
The capsalids primarily rely on an adhesive substance for attachment to the host, but they do have two pairs of anchors, a pair of accessory sclerites, and 14 marginal hooks. Many also have two circular attachment organs at their anterior ends. They also have two pairs of eye spots, but these are not easily discerned in adults. As capsalids reach maturity, their bodies tend to spread out to accommodate their extensive digestive system. Consequently, an infected fish may have capsalids at different stages of maturity that do not appear to be related. The eggs of capsalids usually have long thread-like appendages with adhesive droplets (Figure 7). Mature capsalids and egg masses are often large enough to be seen with the unaided eye.
Clinical Signs and Effects
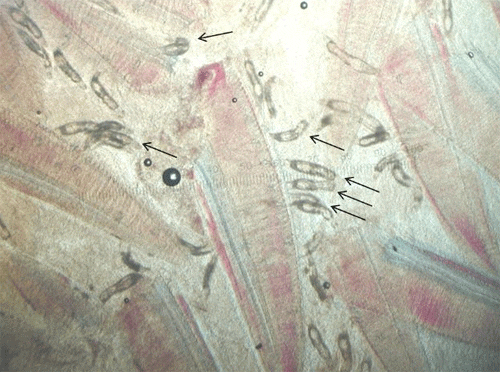
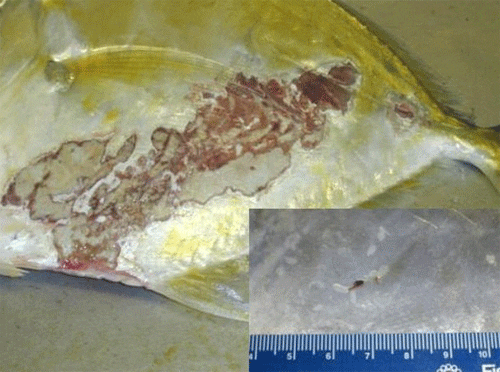
Fishes infested with monogeneans may become lethargic, swim near the surface, have clamped fins, seek the corners of aquaria or the sides of the pond, and have diminished appetite. They may be seen rubbing the bottom or sides (flashing) of the tank. Scale loss may occur where the monogeneans are attached, and the skin may vary in color where the parasites have fed. Heavy gill infestations result in respiratory disease (Figure 8). Gills may be swollen and pale, respiration rate may be increased, and fish will be less tolerant of low-oxygen conditions. Piping (gulping air at the water surface) may be observed in fish in severe respiratory distress. Large numbers of monogeneans on either the skin or gills may result in significant damage and mortality. Secondary infections with bacteria and water molds are common on tissue that has been damaged by monogeneans. In marine fishes, the capsalid monogeneans may infest the skin, eyes, and gills, resulting in extreme irritation to the host. Gray patches and open wounds may appear on the skin (Figure 9), and the eyes may be swollen and appear cloudy. Monogenean infestation should be suspected in sharks when sand grains are stuck to their gills. Sharks will pull sand into their gill chambers in an attempt to rub off the parasites.
Transmission
Transmission of monogeneans from fish to fish is primarily by direct contact. Monogeneans have a direct life cycle, which means that no intermediate host is required for the parasite to reproduce. Adults are hermaphroditic (each organism has both male and female reproductive structures); however, self-fertilization is rare.
The viviparous gyrodactylids produce young that are fully equipped to immediately attach to the host, or they may be carried by the water to another host. These newborns carry several generations of developing embryos. This ability can contribute to population explosions in aquaculture systems, resulting in clinical disease.
In contrast to the gyrodactylids, the eggs of oviparous monogeneans (i.e., Dactylogyridae, Capsalidae, and Ancyrocephalidae) often have appendages that either slow movement in the water column or allow them to be easily trapped in mucus or other organic material. When the free-swimming ciliated larvae (oncomiracidia) emerge from the eggs, they are carried to a new host by water currents as well as by their own movement. The eggs of the capsalid monogeneans are especially sticky and can be trapped in gill mucus, filter media, and substrate. The eggs of all monogeneans are impervious to treatment, so repeated treatments of affected systems are required to kill hatching larvae.
The time required for maturation from eggs to adults is temperature dependent. At water temperatures of 7275F (2225C), only a few days may be required for completion of the life cycle, whereas at water temperatures of 3436F (12C), generation time may be extended to five or six months.
Once immature monogeneans find a host, they crawl on the surface of the hosts body to reach their preferred location.
Management
The best way to manage monogeneans is to avoid introducing parasites to a new system. This can be done by following a quarantine protocol. If quarantine is not possible, a simple way to minimize the introduction of monogeneans and other external parasites is to dip fish in fresh or salt water, depending on the fish species. Dipping saltwater fish in freshwater will reduce the number of many single-celled external parasites, and freshwater fish can be dipped in sea water to accomplish the same goal. Monogeneans that are found on euryhaline fish species are often tolerant of varying salinity, so they are not as likely to be affected by this method. Regardless of the salt concentration used, the minimum contact time is 10 minutes (15 minutes for some monogeneanssee hypersalinity in the section on treatment). However, if the fish roll over before 10 minutes has lapsed, they should be immediately removed from the dip. Dipping fish will not completely eliminate the risk of introducing parasites to an established tank or system, but it may help minimize the numbers brought in. Unfortunately, the sticky eggs of monogeneans are resistant to changes in salinity and are easily transported into the new facility even when fish have been appropriately dipped.
Ideally, fish should be quarantined for at least four weeks before they are placed into a new system. While the fish are in quarantine, perform gill, fin, and skin biopsies to determine whether monogeneans or other gill, fin, or skin pathogens are present. Any parasites identified using biopsy techniques can then be specifically treated. The design of a quarantine system should be very simple so that fish are readily accessible for observation and handling and so that water can be easily changed and treatments easily administered.
A number of chemicals have been used to control monogenean infestations of fish (Table 3). However, treatment of monogeneans must be accompanied by identification and reduction of environmental or husbandry-related stressors, if they are present. As mentioned in the introduction, stressors include inadequate nutrition, poor water quality conditions, over-crowding, antagonistic behavior of other resident fishes, etc. Stressful conditions can not only inhibit the immune system of the fish, but also result in release of cortisol, a stress hormone. In studies of monogenean-infested fish, exposing them to corticosteroids (stress hormones) induced monogenean reproduction.
Take steps to prevent monogenean infestation whenever new fish are to be introduced to a system, even when the new fish appear healthy. Some fishes are capable of mounting an immune response that will prevent monogeneans from reaching lethal numbers. Such fishes may appear normal, but can serve as a reservoir of monogeneans that can infest nave fishes.
Diagnosis
Monogeneans dont live long after a fish dies, so examination of a live or fresh dead fish is best. They may be diagnosed by performing biopsies of fin, gill, and skin mucus and examining these tissues with a light microscope. For monogeneans that live internally, necropsy is required. In order to treat appropriately, it is important to determine which monogenean family is present. For example, prolonged treatment will be required to kill the hatching young of the egg-laying species. Though identification to species isnt necessary to initiate the appropriate treatment, it is important to add to our current knowledge of host/parasite interaction. In the European countries where Gyrodactylus salaris occurs, identification is crucial because its presence must be reported to animal health authorities.
Preservation of monogeneans for identification and future study should include both permanent mounts and 95% ethanol-fixed specimens. Contact a fish health professional for assistance with preservation methods.
Treatment
The treatment of choice for monogeneans for both freshwater and marine fishes is praziquantel; however, praziquantel is not approved by the US Food and Drug Administration (FDA) for use in fishes. Extralabel use of praziquantel for non-food fishes can be considered as long as a valid veterinarian-client-patient relationship exists (see http://www.nrsp-7.org/Legislation/AMDUCA.pdf for information on extralabel drug use in animals and the veterinarian-client-patient relationship). If praziquantel is used to treat monogeneans in ornamental species, its use should be restricted to enclosed aquaria, and any effluent from the treated tank should be run through an activated carbon filter before it is discharged. A common treatment method is to use praziquantel at 25 mg/L in a prolonged bath for 23 weeks. If water changes are performed during the treatment period, the replacement water should be treated to maintain the optimal praziquantel concentration. Praziquantel has also been used successfully to eliminate gill monogeneans in ornamental fishes by adding 2.5 mg/L every other day until a cumulative dose of 10 mg/L is reached, and then repeating the treatment every 14 weeks for a total of 3 treatments. Short baths of 1020 mg/L for 13 hours are often effective for skin monogeneans. Regardless of concentration or contact time used, small monogeneans may be protected by mucus and thus survive treatment. Infested fish should be re-examined 12 weeks post-treatment to determine if treatment should be repeated. Because praziquantel is a selective drug for flatworms, it is the most effective drug for controlling and possibly eliminating monogeneans, and it is safer for the host. However, if praziquantel in the powder form is not prepared properly, it can be very irritating to fish gills and may result in severe gill trauma and subsequent death. Praziquantel is not water soluble but is soluble in alcohol. Only use as much ethyl alcohol as necessary to get praziquantel into solution. The solution should then be mixed thoroughly in a bucket with water from the aquarium to be treated; this mixture can then be added to the aquarium. Praziquantel may also be given orally by mixing it into feed at the rate of 40 mg/kg of body weight per day; the praziquantel-medicated feed should be administered for 11 days.
Hydrogen peroxide has been used successfully for monogenean removal; however, high doses 300560 mg/L (ppm) for 10 minutes are required. Some fishes may be intolerant of such high doses, so hydrogen peroxide should be used with extreme caution. Hydrogen peroxide is approved for use in coolwater food fish and channel catfish for certain bacterial infections; however, it is not FDA approved for treatment of monogeneans (see UF/IFAS Fact Sheet FA157 Use of Hydrogen Peroxide in Finfish Aquaculture).
Formalin, when administered at 30 mg/L (ppm), can also be used; however, vigorous aeration is required to maintain an acceptable level of dissolved oxygen. Note that the 30 mg/L concentration is higher than the dosage recommended for most fish parasites. Re-treatment after 30 days may be required. Sick fish do not tolerate formalin well and all fish should be carefully watched during chemical administration (see UF/IFAS Extension Fact Sheet VM-77 Use of Formalin to Control Fish Parasites). If an adverse reaction is observed, fish should be removed from the treatment tank at once and placed in clean water. Parasite-S, Formalin-F, and Formacide-B are brands of formalin that are FDA approved as monogenean parasiticides for use in all finfish; they, therefore, can be used on food fish as well as ornamental species. Formalin removes oxygen from the water, so vigorous aeration is required during treatment. Also, formalin should never be applied to ponds at dusk.
Potassium permanganate is also moderately effective against monogeneans and is the treatment of choice if columnaris bacteria or water molds have infected damaged tissues. Potassium permanganate can be administered as a prolonged bath at a concentration of 2 mg/L or as a short-term bath (1030 minutes) at a concentration of 10 mg/L. Again, fish must be observed carefully while they are in contact with the chemical, and they should be removed at once if adverse effects are noticed. For more information see UF/IFAS Extension Fact Sheets FA-23 The Use of Potassium Permanganate in Fish Ponds and FA-37 Use of Potassium Permanganate to Control External Infections of Ornamental Fish. Potassium permanganate may be the treatment of choice for pond fish; however, chemical safety and efficacy varies with the organic content of the water to be treated. This chemical is not FDA approved but is an investigational new animal drug (INAD) (see Fact Sheet: Potassium Permanganate INAD 9246).
In marine systems, copper treatments applied at 0.2 mg/L active copper ion for up to three weeks are helpful in controlling but not eliminating capsalid monogeneans. Though copper is reported to be toxic to elasmobranchs such as sharks and rays, it is often used to treat them for parasites. However, copper is lethal to many invertebrates, and some elasmobranchs and other fish species are more sensitive, so it should be used with caution. Copper is not FDA approved, but is an investigational new animal drug (see Fact Sheet: Copper Sulfate INAD 9101).
Another method that can be used for capsalid monogeneans is hyposalinity. In one study, 15 g/L (ppt) salinity or lower for two days eliminated juvenile and adult Neobenedenia melleni. When 15 g/L was maintained for 5 days, the hatching of N. melleni eggs was prevented. It is important to note that some fish species may not tolerate this treatment method.
Use of increased salinity to control monogeneans in freshwater fishes can have variable results. Some monogeneans are more tolerant of saline conditions than others. For example, one study of two gyrodactylid species, Gyrodactylus turnbulli and G. bullatarudis on guppies found that 100% of G. turnbulli were eliminated when guppies were exposed to 25 g/L (ppt) salinity for 15 minutes. However, the same treatment removed only 72% of G. bullatarudis. This same study reported that increasing salinity from 0 to 3 g/L resulted in an increase in numbers of gyrodactylids. The authors reported the fish produced more mucus in response to the increased salinity; the monogeneans fed on the increased mucus and were also able to adjust to the salinity change. In contrast, some freshwater monogenean species are able to live for several days in full-strength seawater (3035 g/L) but are not able to reproduce during that time. This wide variability in salinity tolerance of the different monogenean species found on freshwater fishes means the use of increased salinity may not be effective in controlling them.
In one study that examined biocontrol of capsalids, the cleaner wrasse Labroides dimidiatus was effective at eating large capsalid monogeneans from the host fish, banner wrasse Hemigymnus melapterus. However, smaller capsalid monogeneans were not consumed, and it is not known if cleaner wrasse themselves are susceptible to infestation by monogeneans. Whittington and Deveney (2011) suggested that cleaner wrasse may act as a source of monogenean infestation at cleaning stations.
A final method of control is to empty, dry, and disinfect tanks or ponds before restocking them. Except for this method, it is very difficult to completely eliminate monogeneans once they are introduced.
Summary
Monogeneans are found on fresh- and saltwater fishes throughout the world. They have a direct life cycle and can reproduce in a wide range of temperatures. The hook-like structures of monogeneans are used to attach to the fish. Monogenean infestations cause irritation and excessive mucus production and create an opening for bacterial invasion. A few monogeneans on a healthy mature fish are not usually significant; however, moderate numbers can cause significant mortalities. When fish are exposed to environmental or behavioral stressors, the potential damage from monogeneans is greater. Prevention of monogenean infestations by following appropriate quarantine is preferable to treatment of the parasites after they have become established in a system.
December 2012


