
Mariculture is a form of rearing aquatic organisms for commercial purposes, either in an open coastal ecosystem or in a controlled marine ecosystem. It is an important aquaculture activity in the Asia-Pacific region. There are over 40 marine fish species commonly cultured, such as groupers (Epinephelus spp.), snappers (Lutjanus spp.), Asian seabass (Lates calcarifer) and golden pompano (Trachinotus blochii). They are typically cultured in open floating net-cages along the Asia-Pacific coastal areas, with an annual production of approximately one million tonnes.
 Traditional marine fish farming in South-East Asia. Different species of fish with overlapping generations are cultured at one farm site. The lack of good health management practice, such as high stocking density, feeding trash fish and poor sanitation, has led to a high incidence of diseases. |
Under natural environmental conditions, coastal waterways are free of obstructions. However, the placement of floating net-cages along these waterways has created ‘artificial islands’, resulting in the congregation of a diverse biological community comprising both vertebrate and invertebrate organisms. Therefore, one would expect to find a similar congregation of bacteria, viruses, fungi, parasites and other pathogens within this newly- created ecosystem, as well as the natural occurrence of other wild aquatic organisms.
Mortality of a large number of fish is seldom observed in the wild and, when it does occur, it is most likely to be due to sudden environmental deterioration. However, in the confined net-cage environment, mortality is often seen. Signs include abnormalities in behaviour, darkened body, exophthalmia (pop eye) and ulcerations on the fish body. There are many causes of fish mortality in the confined net-cage environment but possible causes for these disease outbreaks are pathogenic parasites.
Parasites are invertebrate organisms; some are free-living and can become opportunistic parasites; others require hosts for their survival and reproduction, and these are referred to as obligate parasites. Both opportunistic and obligate parasites are found in fish hosts but most parasitic diseases in fish are generally caused by obligate parasites.
Types of parasitic diseases
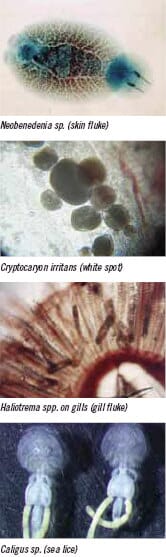 Examples of some of the parasites that cause diseases in marine fish in the Asia-Pacific region. |
Most apparently healthy fish usually harbour various parasites but at low numbers, either on or in their bodies. The low number of these parasites generally causes little or no harm to the fish. However, when the number of parasites per fish increases significantly (the natural parasite-host balance becomes broken) due to overstocking, changes in water temperature or salinity that are favourable to the reproduction and growth of parasites, or that cause a reduction in fish immunity (due to these stressful conditions), parasitic disease outbreaks often occur.
Parasitic disease and other pathogens are interrelated. For example, bacterial and viral diseases can weaken fish and make them more susceptible to parasitic infestation and vice versa. In an aquatic ecosystem, where the health conditions of cultured fish are not easily observed, proper care of the fish and their environment are of the utmost importance. This is to help the natural immune system of the fish react and keep the pathogens in check.
A large variety of parasites have been reported in cultured marine fish. Some of these parasites have caused serious disease outbreaks in farmed fish resulting in significant financial losses to fish farmers. Parasites either cause major disease outbreaks in cultured fish or rather contribute to a chronic sub-clinical effect. In general, the fish are most susceptible at the early stages, particularly at the hatchery and nursery stages of the culture cycle when fish are small.
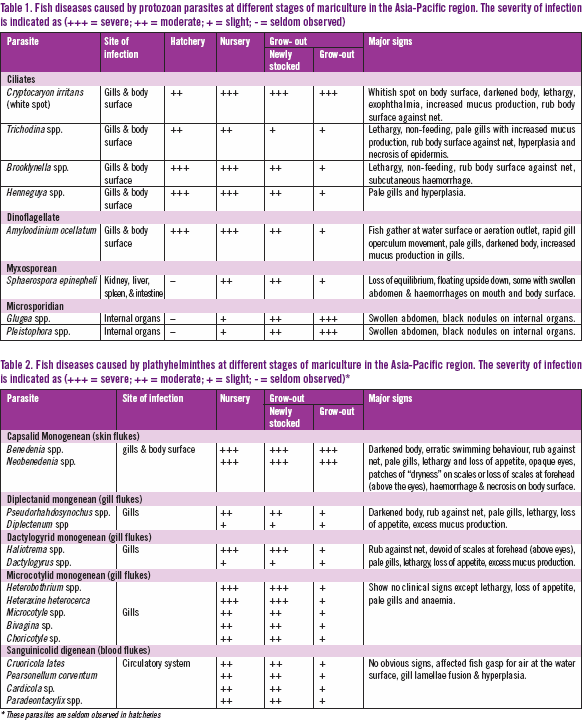
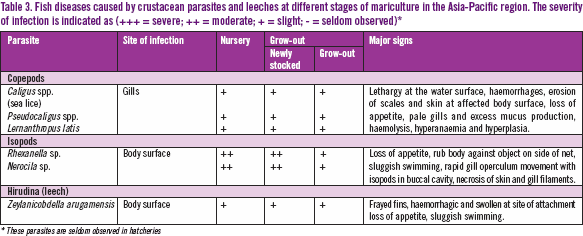
Parasitic organisms affecting cultured fish can be grouped into three main groups, namely, protozoa (Table 1), plathyhelminthes (e.g., flatworms & roundworms; Table 2) and crustaceans (Table 3). At each stage of the culture cycle, the pathogenicity of a particular pathogen on a fish host may differ. For example, the protozoa Amyloodinium ocellatum is very pathogenic to fry and fingerlings at the hatchery and nursery stages. At the initial stage of the grow-out phase of net-cage culture, the newly stocked fish become increasingly susceptible to parasitic diseases caused by capsalid monogeneans.
Many of these parasites can cause disease outbreaks and significant financial losses. Severity of parasites at various stages of the growout period and the disease signs are presented in Tables 1–3.
Entry of parasites to the culture system
Many species of marine fish are now produced in the hatchery for culture in ponds or net-cages, but some are still wild-caught. At the nursery stage, these fish are placed in cement tanks, ponds or netcages for some time, before being transferred to grow-out cages.
It is very unlikely that fish nursed in cement tanks would have monogenean infections but they could be infected with protozoa. If the fry are nursed in ponds or nursery cages, they could acquire protozoa and monogeneans, as well as other pathogens. The wild-caught fingerlings could have been infected with pathogens before stocking. Fingerling suppliers may also combine various batches of fish from different sources so as to have sufficient numbers of fish for distribution.
However, an infected batch will spread disease to the others. Furthermore, net-cage farming is an open system whereby fish reared in the cages are in close proximity with wild fish which may transfer pathogens to those inside the cages. Also, in this region, trash fish are commonly used as feed which can act as a source of parasites.
One of the major problems in implementing control measures to prevent parasitic infection in net-cage fish farming in Asia is the overlapping generations (and species) of fish. There are no breaks in the production cycle or fallowing before the next batch of fish is stocked. As a consequence, naïve fish introduced into the farm would likely be infected with one or more species of parasite which already exist on the farm. Therefore, the fish farm itself is a reservoir for parasites.
Given the above scenario, how would one then be able to control parasitic infections in a farm? It is not possible to have a parasitefree environment; however, one can implement preventative and control measures to limit the populations of parasites in fish and to minimize the associated diseases. Fish infected with few parasites show no ill effects. Usually, parasitic diseases are caused by a high density of parasites. Very often, concurrent secondary bacterial infections occur, imposing an additive or synergistic effect on fish mortality.
A successful parasitic diseases control programme consists of the selection of healthy fish, quarantine, good husbandry practices, preventive measures, correct diagnosis and, if necessary, therapeutic treatment. Unfortunately, under farming conditions in the Asia-Pacific region (especially in South East Asia), farmers often do not have technical expertise in proper health management at farm level under local farming conditions. An experienced farmer might know that the fish are not well but may not know the cause and what needs to be done. Technical support for accurate diagnosis and for recommending appropriate treatments is generally lacking.
As outlined in Part 1 of this article, a variety of parasites infect fish at different stages of the production cycle. The pathogenicity of each parasite differs for each species of fish, as well as for each stage of the growth cycle. For example, the protozoa Amyloodinium ocellatum is very pathogenic to fry in the hatchery, but may not have any effect on adult fish. Furthermore, most parasites (except for the sanguinicolid digenean blood flukes) have direct life cycles, i.e., they do not require an intermediate host for development to maturity.
It is impossible to totally avoid parasites as they usually exist as part of the aquatic ecosystem. A few parasites on a fish usually cause no harm. Disease occurs only when the parasite-host balance is upset.
 1. Prophylactic treatment given during fish grading  2. Bath treatment for control of skin flukes using a tarpaulin sheet for large number of fish |
Many parasitic diseases reported in farmed fish show similar disease signs (see Table 1 in Part 1). Therefore, it is difficult for farmers to identify what is the primary cause of the disease. As outlined in Part 1, the parasites in marine fish can be broadly grouped into the following: protozoa, diplectanid and dactylogyrid monogeneans (on the gills), capsalid monogeneans (on the body surface), sanguinicolid blood flukes, parasitic copepods, parasitic isopods and leeches. Usually only protozoa (particularly Cryptocaryon irritans and Trichodina spp.) and capsalid monogeneans on the body surface (particularly Neobenedenia spp.) are problematic and pathogenic to fish, especially to newly stocked juveniles in cages.
Considerations regarding drug/chemical treatments
It must be emphasized that prevention is the key and treatment is a last resort. Very often, when disease occurs in the farm, chemotherapeutic treatment is too late and often ineffective.
In most countries, very few drugs and chemicals have been registered for treatment of food fish. Indeed, many biocides (e.g., malachite green) are actually banned from use in most countries (including all the major fish-importing countries) and severe measures are taken against exporters of fish and shellfish that contain residues. Therefore, the drugs/chemicals used must be safe to fish, the environment and humans. For food safety, guidelines on the withdrawal period for each chemical must be observed.
When disease occurs, treatment is usually administrated by bath immersion. However, chemicals (e.g., copper sulphate) added to the water in recirculation systems may disturb the biological filter system and cause erosion to equipment. Frequent addition of chemicals to the water can also lead to the induction of resistance in parasites.
This article will not discuss specific treatment recommendations as such information is available from many other sources and drug/chemical usage is always subject to local regulations. In general, freshwater, hydrogen peroxide and formalin are commonly used as bath treatments against protozoa and capsalid monogeneans.
Tips for bath treatment of fish
• For safety reasons, always first try the chemical, at a given dose and treatment time, with a small number of fish. Fish of different species and sizes under different water conditions (salinity, alkalinity and temperature) may well react differently. In general, lower water temperature requires a longer treatment duration and vice versa.
- Follow the correct dose and treatment time. Pay close attention to concentration of the active ingredient and adjust the dose accordingly if the chemical is not pure (<100% active).
- Add the chemical to a small portion of the water in a small container and make sure it is dissolved completely before use. Then pour this ‘concentrate’ into a fish holding tank/container to reach the desired final concentration and mix well before placing the fish into it.
- Withhold feed for 8-24 hours depending on the fish size.
- Treat during the coolest part of the day.
- Monitor water oxygen levels before, during and after treatment; if necessary, aerate as required.
- Keep a close eye on the fish during treatment and be prepared to stop treatment immediately if adverse reactions (e.g., gasping for air, strange swimming behaviour, etc.) are noted.
A strategy for integrating treatment into routine farm activities
 3. Skin lesions caused by skin flukes and subsequent rubbing against fixed objects in the net-cage in a grouper (top) and red snappers (bottom) |
With a general understanding of multi-species fish farming and their diseases, a strategy for disease control can be developed and integrated into routine fish farm activities. The following steps are recommended for implementation:
- A supervisor/manager/health monitor should be trained and routinely updated on disease diagnosis and control measures.
- Specific net-cages (ideally set apart from the main farm area) should be set aside as a quarantine area for new fish arrivals.
- After placement in the net-cages, newly-arrived fish should be observed closely; some fish species are more susceptible than others.
- Within the first week, susceptible fish (such as groupers and red snapper) should be given a prophylactic treatment.
- Prophylactic treatment consists of immersing fish for 10 - 30 minutes (depending on the tolerance of the fish species and their size) in freshwater mixed with formalin (100-200 ppm).
- Grading fish should be routinely undertaken within the first 3-4 months of stocking, and prophylactic treatment (as mentioned above) should be incorporated into the process of fish grading (see photos 1 and 2).
- When high mortality occurs, moribund fish should be sent to a fish health laboratory to determine the cause(s) of mortality.
- In the event that a parasitic disease outbreak occurs, a second freshwater treatment should be carried out three days after the first one, followed by a third one five days later.
The above steps should be incorporated into routine fish grading activities throughout the production cycle as parasitic diseases could occur, especially with capsalid monogeneans, at any time of the year. The purpose of these steps is to reduce the rapid build-up of monogenean parasite populations.
An experienced supervisor or farm worker would know that a disease outbreak is about to occur if observations indicate the fish are not well (from a reduction or a cessation of feeding), are dying, have abnormal swimming behaviour (such as swimming slowly on the water surface), or have haemorrhages, red boils or patches of scales showing dryness or lesions from rubbing (Photo 3), typically due to skin irritation caused by monogeneans. These signs may appear concurrently in one or more net-cages with the same species of fish. If the farmers are slow to implement treatments, fish of the same species in other net-cages could also show similar signs a few days later. High mortality of fish could then be expected, even if one (belatedly) starts treatments.
The need for safe and effective anti-parasitic medicines
Although the freshwater (with or without formalin) prophylactic treatment is very effective against most of the parasites, it is a tedious process. Also, freshwater is not readily available to most marine fish farms and a large amount has to be transported to the farms whenever it is needed.
Current observations of floating net-cages indicate that capsalid monogenean infections are the most serious and pathogenic amongst all parasitic diseases in the Asia-Pacific region.
Examination of red snapper after freshwater treatment during a capsalid monogenean outbreak indicates that very rapid re-infection occurs (Figure 1). In this study, all monogeneans recovered were very young, indicating infection of the monogeneans occurred after they hatched from the eggs. The capsalid monogeneans are not host specific for fish but rather infect all fish species in the surrounding net-cages. Some fish species are more susceptible than others, resulting in greater frequencies of parasitic disease outbreaks for that fish species.
A number of “microbiocides” or other compounds have been tested and/or used against fish parasites. They include various disinfectants (such as chloramine-T and formalin), hydrogen peroxide, anthelminthics (such as praziquantel and fenbendazole), organophosphates (such as dichlorvos), pyrethroids (such as deltamethrin), avermectins (such as ivermectin) and chitin synthesis inhibitors (such as diflubenzuron).
More recently, there have been several papers published by Japanese scientists on the effect of caprylic acid, either administered orally or by immersion, on ecto-parasites, including Benedenia. Praziquantel is available on the market in some countries against skin flukes. While certain efficacy has been shown, palatability (bitter taste) and high cost are problematic for its application. For monogenean infestation, freshwater treatment is the safest and most effective method available at present but the process is indeed tedious. A safe, effective and economical anti-monogenean drug needs to be developed. Ideally, the drug could be incorporated into the feed for routine prophylactic treatment for a broad range of parasites in all species of fish.
Apart from chemotherapeutic treatments, emphasis should be given to health management practices, including biosecurity and optimization of the culture environments. The poor but still common practices of overstocking, using trash fish, mixing species and overlapping generations in the same cage must be stopped or improved. In recent years, things have improved a little by optimization of cage placement to reduce the incidence of parasitic diseases.
Like the control of bacterial and viral diseases, vaccination is an attractive and sustainable (but challenging) option to tackle parasitic pathogens. Such research is ongoing at several locations; for example, the development of Cryptocaryon and sea lice vaccines. While progress has been slow due to the complexity of host immunity and pathogen interactions, we remain hopeful for a breakthrough.
In summary, freshwater treatment is the safest and most effective method available at present but the process is tedious. A safe, effective and economical anti-monogenean drug needs to be developed. Ideally, the drug could be incorporated into the feed for routine prophylactic treatment of all species of fish.
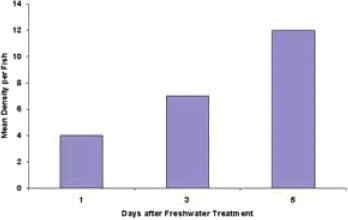
Figure 1. Recurrent infection of capsalid monogenean in red snapper after freshwater treatment
Suggested Reading
APEC/SEAFDEC 2001. Husbandry and health management of grouper. APEC, Singapore and SEAFDEC, Iloilo, Philippines. 94 pp.
Chong, Y.C. and T.M. Choo, 1986. Common diseases of marine foodfish. Fisheries Handbook No.2. Primary Production Department, Ministry of National Development, Republic of Singapore. 34 pp.
Intervet International B.V. 2003. Guide to veterinary antimicrobial therapy. 4th edition.
Leong, T.S., 1994. Parasites and diseases of marine finfishes in Southeast Asia. Universiti Sains Malaysia. 25 pp.
Nagasawa, K. and E.R. Cruz-Lacierda (eds), 2004. Diseases of cultured grouper. Southeast Asian Fisheries Development Center, Aquaculture Department, Iloilo, Philippines. 81 pp.
Woo, P.T.K., D.W. Bruno and L.H.S. Lim (eds)., 2002. Diseases and disorders of finfish in cage culture. CABI Publishing, UK. 354 pp.
Further Information
To continue reading the full article, including tables, click here (PDF)
September 2006

