Against a backdrop of long term declines in stock levels of Atlantic salmon (Salmo salar L.) throughout its range, stock levels in Ireland and the NE Atlantic since the 1970s (ICES) have given rise to serious concerns for the status of the species.
This has resulted in conservation measures being introduced and strengthened to include restrictions in existing fisheries, closures of mixed stock fisheries and the introduction of carcass tagging and quota systems (O'Maoileidigh et al., 2004). It has also led to increased interest in gaining a better understanding of the factors underlying the current trends (Peyronnet et al., 2007).
Significant declines in sea survival and reduced returns to the coast and rivers of Atlantic salmon in recent decades have been recorded in Ireland (Salmon Management Task Force Report (Anon., 1996); O'Maoileidigh et al., 2004). The reasons for the reduced sea survival remains unclear and speculation has covered such issues as global warming effects ( [Friedland et al., 2000] and [Friedland et al., 2005]), changes in locations or availability of prey species, loss of post-smolts as by-catch in pelagic fisheries, increased fishing pressure, habitat changes and sea lice infestation (Finstad et al., 2007). However, despite many years of study, processes contributing to the high mortality of juvenile Atlantic salmon between ocean entry and the first winter at sea remain poorly understood (Jones, 2009).
In order to investigate if lice infestations were a significant factor in early marine mortality of Irish salmon smolts and to measure the inter annual variation in the impacts of early lice infestations on sea survival the Marine Institute has been undertaking a long term study of lice infestations in outward migrating salmon smolts. The goal of this study is to attempt to measure the impact of early infestation of outward migrating salmon smolts with the salmon louse, Lepeophtheirus salmonis Kryer in established ranched strains. The study is based at the Marine Institute research facility in Burrishoole in County Mayo (Fig. 1, location map), which is an index catchment with full upstream trapping. The Burrishoole ranched stock is an established strain which has been studied for over 30 years (Piggins and Mills, 1985) and there is considerable historical data on its marine survival and return rates. Studies are also under way on ranched stocks at other locations in Ireland.
Materials and Methods
Experimental Design
By treating experimental batches of tagged fish with a prophylactic dose of SLICE, a commercial sea lice therapeutant, prior to release the fish can be protected from infestation with the salmon louse for up to 9 weeks ( [Stone et al., 2000] and [Copley et al., 2007]). The active ingredient in SLICE is emamectin benzoate. It is an animal medicine licensed for use in Ireland as a treatment for sea lice infestation in salmon. Since 2001 the Marine Institute has released treated and control groups of experimental ranched smolts over a number of years and recorded their subsequent survival and return rates as grilse and multi sea-winter fish to the coast and their release catchments. Treated fish are protected from sea lice infestation in their early weeks in the sea and therefore can be expected to be free of any adverse impacts on their survival related to early lice infestation. As salmon smolts are known to migrate quickly out of the bays and into the open sea treated smolts will have moved well offshore before the protective effects of the SLICE treatment have worn off. Studies at Burrishoole have shown that salmon smolts have moved into coastal waters within 48 h (Moore et al., 2008) and post-smolt recapture data ( [Shelton et al., 1997] and [Dadswell et al., 2010]) has shown that smolts from the study area have travelled a distance of over 700 km in 7 weeks and are in an area north of Scotland and west of Norway. By comparing their survival and return rates with control fish, which do not enjoy this protection it is possible to differentiate any additional mortality associated with lice infestation in the first 6 to 8 weeks post migration.
Fish Stocks
The stock used in the study is the Burrishoole grilse stock. This stock has been line bred in an ongoing experimental ocean ranching programme since the early 1970s ( [Piggins and Mills, 1985] and [Cotter et al., 2000]). In each release experimental groups of smolts were split into two approximately equal groups, one treated and one control. The treated groups were administered SLICE as an in feed preparation at the rate of 50 ?g/kg/day for 7 days. Treatment was completed in approximately 7 days before the release date of the smolts. Control groups were fed either with food mixed with a placebo or, in certain years, with untreated food.
Samples of treated food were retained and analysed to ensure appropriate inclusion rates and samples of both treated and control fish was taken for flesh analysis. Fish samples were taken 2 days post-feeding to ensure the guts were voided of medicated feed. Flesh analysis for emamectin benzoate was carried out by accredited laboratories to ensure a therapeutic dose was present in the treated groups prior to release.
Tagging
Experimental batches of fish were all tagged with coded wire tags. Pre-smolts were microtagged according to the methods of Browne (1982). Each magnetised microtag had a specific code which identified the release group and stock of the fish. A 1 mm long magnetised tag, etched with a specific batch code was injected into the nose cartilage of the juvenile fish. The code identifies the origin and release circumstances of any fish subsequently recaptured. All fish were anaesthetised when tagged. The adipose fin was removed to facilitate the identification of these fish in the recovery programme. A quality control check was made on the tagged fish to ensure that the tag has been correctly magnetised. Tagging mortality and tag loss were also estimated and subsequent analyses were based on the numbers of fish migrating rather than the number of fish tagged.
Tag Recovery
Information on capture location and return data of the experimental groups was gathered as part of an ongoing Irish national coded wire tag recovery programme ( [Browne et al., 1994] and [O'Maoileidigh et al., 2004]). Catches from coastal commercial fisheries (drift nets, draft nets, etc.) were monitored at 15 major salmon landing ports in Ireland. These fisheries operate between May and July inclusive and catches were scanned consistently during this period. Over 50% of the catch landed in Ireland is sampled for tags each year. The number of tagged salmon taken in these fisheries (raised data) was estimated by multiplying the actual number of tagged salmon in each area by the ratio of the total declared salmon landings in these areas to the sample size examined. An adjustment for non-catch fishing mortality due to losses from nets and non-reporting of catches was also applied.
Complete upstream and downstream trapping facilities at the Marine Institute hatchery, situated on the Burrishoole river system in Co. Mayo, ensured an accurate count of the numbers of tagged adult salmon returning to the hatchery location. The number of fish entering the river was derived from total trap data and angling for the Burrishoole system. For fresh water, the percentage return was calculated using the actual number of tags recovered divided by the number of fish migrating.
Release Groups
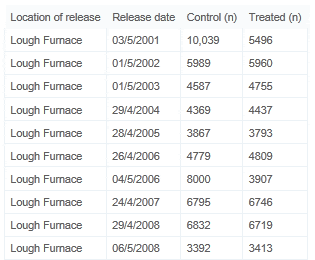
Results for a total of 10 releases over a period of 9 years, from 2001 to 2008 are presented. There were two releases in each of 2006 and 2008. Details of release dates and the size of groups are given in Table 1. Ranched release groups were released as 1+ year old smolts into Lough Furnace, a tidal lake immediately downstream of the Marine Institute hatchery at Burrishoole, County Mayo.
Details of Release Dates, and Numbers Migrating for All 10 Groups.
Data Analysis
A sign test was calculated on the observed returns of treated and non-treated salmon over the entire test period to determine if treatment improved potential of salmon returning. Two way contingency tables were used to calculate expected returns for comparison against observed returns for each yearly pair of treatment and control batches using the chi-squared test. To investigate in more detail changes in the number of returns of treated and untreated salmon over the experimental period, and the differences and similarities in these changes between the treated and untreated batches, an analysis of co-variance (ANCOVA) was used (Sokal and Rohlf, 1995).
Results
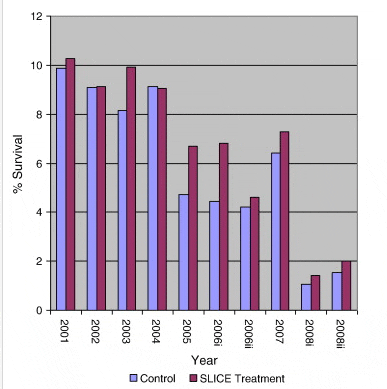
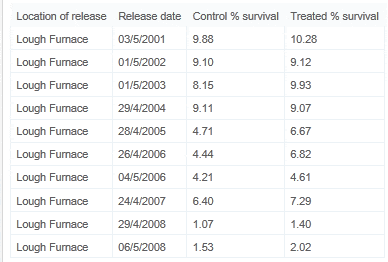
The actual numbers of returning fish recovered for each experimental release is shown in Fig. 2. Percentage survival for the same groups is shown in Fig. 3. A trend of decreasing survival rates in both treated and control groups over time can be clearly observed. Percentage survival ranged from a maximum of just over 10% in the 2001 release treated group (10.28%) to a minimum of just over 1% in the 2008 early release control group (1.07%). The maximum difference in percentage survival between treated and control groups (2.38%) was recorded in the early release group in 2006 when the percentage return for the treated group was 6.82% as against 4.44% in the control group. Percentage survival rates for all groups are outlined in Table 2.
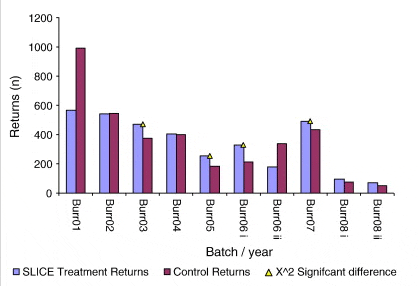
Details of Percentage Survival for All 10 Groups
A sign test was calculated on observed returns of treated and non-treated salmon (n = 10). In nine instances a greater proportion of treated than non-treated salmon returned, which represents a significant departure from the expected binomial equality at p < 0.05.
Chi-squared tests of independence showed significant differences in treated and non-treated returning and non-returning rates in four of ten instances (in 2003, X2 = 8.98 p < 0.005; in 2005, X2 = 13.70 p < 0.001; in 2006(i) X2 = 25.64 p < 0.001 and; in 2007 X2 = p < 0.05).
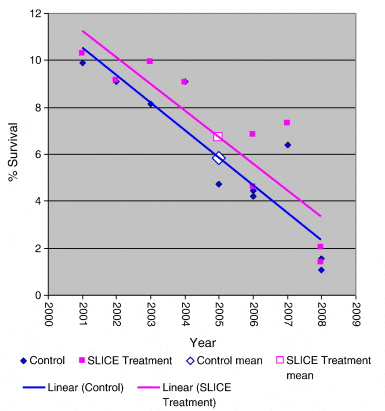
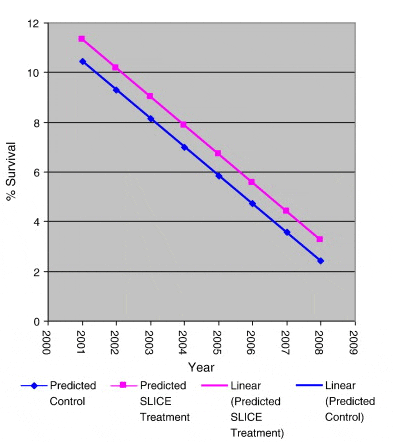
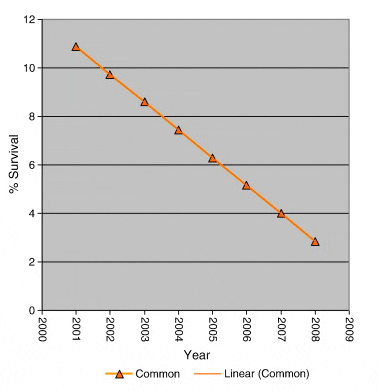
Clear declines in returns in both treated and non-treated batches were apparent over the experimental time period, Fig. 4. An ANCOVA was used to assess relationships between these declining rates. Independently regression lines of the declines in returns were extremely significant (p > 0.001; n = 10 for each), however no difference between the mean returns was found (analysis of variance, n = 20), Fig. 4. A common regression of the two sets (Fig. 5) was extremely significant (p < 0.001; n = 20) though there was no difference between the rates of decline between treated and non-treated returns (n = 20) or between their instantaneous returns when corrected to a common decline rate (Fig. 6) (Sokal and Rohlf, 1995).
Discussion
While treatment with SLICE generally resulted in a higher percentage return than the untreated control group (9 out of 10 cases, sign test) in the majority of releases, six out of ten, this difference was not significant when compared against the expected number of returns (contingency table chi-squared tests). In 2006 the early release group (Table 2) showed the greatest difference in percentage survival, which was extremely significant, however the difference in return in the later release group between treated and control batches was not significant. Over the period of the study the relationships between rates of return for treated and control batches exhibit similar trends.
The difference in percentage survival between the treated and control groups is not significant (ANCOVA) but the fact that the treated groups have higher survival in nine out of ten cases (sign test) is. These results are consistent with the expectation that a reduction in potential parasite load on outwardly migrating smolts, such as would be conferred by protection against sea lice infestation for a period of up to 9 weeks should contribute to increased fitness and survival potential. The results over the study period would suggest that the level of infestation pressure by L. salmonis experienced by the outwardly migrating smolts was not of a level to be a consistently significant source of additional marine mortality because no significant difference in survival rates was found between treated and unprotected groups.
It is well recognised that large numbers of mobile L. salmonis can cause host morbidity and death (Wagner et al., 2008) and natural levels of L. salmonis on wild salmon returning to the Irish coast are known to have a mean abundance of more than 10 mobile lice per fish and a prevalence in excess of 90% (Copley et al., 2005). Skilbrei and Wennevik (2006) found similar survival rates in treated and untreated groups of smolts released in western Norway in May 2003 but found significantly better survival in the treated group released in June of the same year. Glover et al. (2004) suggested that there may be a genetic susceptibility component to differences in infestation rate observed between five different stocks of Atlantic salmon, three wild and two farmed. Finstad and Jonsson (2001) have reported very large differences between treated and untreated groups in Norway. They reported treated groups having recapture rates of 0.9% as against 0.03% in unprotected fish. Differences of this magnitude were not recorded in this study and minimum survival levels were always in excess of 1%.
The highly significant trend observed in both treated and untreated groups of a decline in percentage survival from values in the region of 10% survival in the 2001 releases to values ranging from just over 2% to 1.07% in the 2008 releases is of great concern. Both treated and control groups share a common rate of decline (Fig. 5) and there is no difference in their returns when corrected to a common decline rate (Fig. 6) clearly demonstrating that the long term decline rate is common to both groups. This highly significant decline in marine survival over the study period is independent of whether the fish were treated to protect against infestation with sea lice or not.
Conclusions
The results to date show a strong and significant trend in increasing marine mortality of Atlantic salmon originating in the study area. They would also point to infestation of outwardly migrating salmon smolts with the salmon louse (L. salmonis) as being a minor and irregular component of marine mortality in the stocks studied and not being implicated in the observed decline in survival rate.
Further ReadingYou can view the full report and list of authors by clicking here. |
February 2013




