Aquaculture is still the fastest growing food producing sector, compared to other food commodities with an annual increase of approximately 12% (FAO, 2009). The use of antibiotics in aquaculture as disease prevention and growth promotion may introduce potential hazard to public health and to the environment by the emergence of drug-resistant microorganisms and antibiotic residues. Furthermore, the normal microbial flora in the digestive tract, which is beneficial to fish, is also killed or inhibited by oral chemotherapy (FAO/WHO/OIE, 2006). With increasing demand for environment friendly aquaculture, the use of probiotics in aquaculture is now widely used instead of chemotherapy and antibiotics to increase safety protein production for human. Probiotics include bacteria and yeasts, the beneficial role of yeasts being of particular interest because they represent an important source for nonspecific immuostimulants as β-glucans (Sahoo and Mukherjee, 2002), chitin (Vecchiarelli, 2000), nucleic acids as well as mannan oligosaccharides (Li et al., 2004) and also yeast act as growth promoters (Li and Gatlin, 2005) of various fish species. The objective of this study was to investigate the effects of three different levels of two commercial probiotics, on the growth performance, feed efficiency, immunity and carcass composition of catfish fingerlings.
Materials and Methods
The experimental feeding was carried out during 2010 in the wet lab. of Central Laboratory for Aquaculture Research, Abbassa, Abo Hammad, Sharqia Governorate, Egypt. The study aimed to investigate the effect of dietary graded levels of Super Biobuds and Bioyeast on the performance, feed utilization, blood constituents, and body composition, of catfish (Clarias graiepinus) fingerlings.
Experimental facilities: Twenty one glass aquaria with dimensions of 60 x 75 x 50 cm (water volume 180 l/each) were used at triplicates/treatment. Catfish fingerlings were obtained from Kafr-Elsheikh Governorate, Egypt. After arrival, fish were kept under the same environmental conditions and placed in fiberglass tanks for 2 weeks as adaptation period to alleviate stresses during transportation and to be adapted to the new conditions. Fish were fed a control diet containing 30 % crude protein for two weeks, during this period healthy fish of the same weight replaced the dead ones. Fifteen fish were kept frozen at – 20 °C for proximate analysis at the start of the experiment. After 14 days adaptation period, 210 fish of the same initial body weight (16 g/fish) were selected and randomly distributed into 7 experimental treatments (2 probiotics x 3 levels of each + control) in triplicates. Each aquarium was supplied with compressed air via air-stones using aquarium air pumps. Settled fish wastes with one half of aquarium's water were removed daily by siphoning and water volumes were replaced by dechlorinated aerated tap water from the storage tank. Water temperature range was 25 – 27 °C. Each diet was given to the fish at a rate of 3 % of live body weight twice daily at 9 a.m. and 1 p.m. The appointed quantity of diet was offered to the fish 6 days a week throughout the experimental period (90 days). Fish in each aquarium were weighed biweekly and the amounts of the required feed were readjusted according to the actual body weight. The dead fish was daily recorded and removed. At the end of study, fish were individually weighed and their lengths were measured.
Feed additives: Two preparations of commercial probiotics were used as sources of Lactobacillus acidophillus, Streptococcus faecium, Lactic acid bacteria, Aspergillus oryzae fermentation and bacterial nutrients, yeast (Saccharomyces cerevisiae to test their effects on the growth performance and feed utilization of catfish (Clarias graiepinus) fingerlings. The composition of these probiotic preparations, as claimed by the manufacturers is:
Super Biobuds (P1): It is active dried yeast culture composed mainly of beneficial yeast (800 million CFU/g live cell yeast, Saccharomyces cerevisiae, 6%), extruded wheat middling (45%), calcium carbonates (48.5%), soybean oil (0.5%), digestive enzymes, essential amino acids, vitamins, minerals and immune stimulants.
Bio-yeast (P2): It is dried product composed mainly of yeast culture, beneficial bacteria, digestive enzymes, vitamins, chelated minerals, amino acids and immune stimulants (Saccharomyces cerevisiae 5 billion cells / g, Lactobacillus acidophilus 5 billion cells / g, Streptococcus faecium 5 billion cells / g, Lactic acid bacteria 2.0%, Aspergillus oryzae fermentation 27.5%, yeast fermentation extract yeast fermentation extract 33.5%).
Experimental diets: The probiotics (P1 and P2) were applied in catfish diets at three levels, being 0.5, 1 and 1.5 g/kg diet. The control group received the basal diet free of probiotic supplementation. All experimental diets (Table 1) were formulated to cover all nutrients requirements of catfish (Table 2) as recommended by National Research council (NRC, 1993). The dietary ingredients (from the local market) were finely ground and weighed according to their percentage and mixed together, then water was added to each diet to be easily pelleted by pressing through 1 mm diameter pelleting unit. The pellets were dried in a drying oven at 60 oC for 24 hours and stored at – 4 oC until use during the trial to avoid oxidation and rancidity.
Table 1: Composition (g/100 g) of the diets used in the experiment.
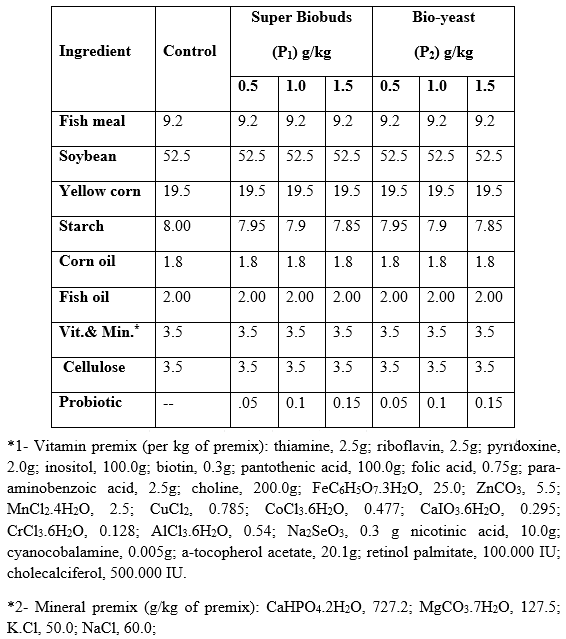
Table 2: Chemical composition (% on dry matter basis, determined according to AOAC (1990)) of the experimental diets.
Experimental conditions: Fish were reared in fresh water. Temperature, pH, dissolved oxygen and photoperiod values were 27°C, 8.8, 6.8 ppm and 12/12 hours light/darkness, respectively. Water exchange rate was 30 % daily of the total volume of rearing water.
Proximate chemical analysis of fish body: After growth trial, the fingerlings at the beginning of trial, and the whole fish body collected from each treatment were analyzed according to the standard methods of Association of Official Analytical Chemists (AOAC, 1990) for moisture (using drying oven GCA, model 18 EM, Precision Scientific group, Chicago, Illinois, USA), protein (using a microkjeldahl apparatus Labconco, Labconco Corporation Kansas, Missouri, USA), total lipids (using Soxhlet apparatus Lab. Line Instruments, Inc., Melrose Park, Illinois, USA), and ash (using a muffle furnace Thermolyne Corporation, Dubuque, Iowa, USA).At the end of the feeding period, fish as samples were weighed and killed. To obtain a homogenous material for chemical analysis, five fish carcasses of each group were homogenized by a mixer and stored at -20oC until analysis. Samples of the homogenized fish carcass materials were taken for the determination of dry matter.
Performance parameters: Catfish fingerlings were weighed biweekly during the experimental period (90 days). Total weight was determined to the nearest gram and the total length to the nearest centimeter. The performance parameters including average daily gain (ADG), relative body weight gain (RBWG%), specific growth rate (SGR), feed intake (FI), feed efficiency ratio (FER), feed conversion ratio (FCR), protein intake (PI), protein efficiency ratio (PER), fat intake (FaI), protein deposition (PD), fat deposition (FaD), protein productive value (PPV%), and condition factor (K) were calculated according to Jauncey and Rose (1982). Also, hepato-somatic index (HIS) was calculated.
-Gain =Final fish weight (g) – initial fish weight (g).
-Gain % = Gain of fish (g) / initial weight of fish (g) X 100.
-ADG = Gain (g) / time (day).
-RBWG % = {ADG / Initial weight of fish (g)} X 100.
-SGR = 100 X {(In W2 – In W1) / T}
Where........... W2 is the final weight of fish (g).
Where........... W1 is the initial weight of fish (g).
In is natural log.
T is the period (day).
-FCR = Feed intake (g) / Weight gain (g).
-FER % = [(Weight gain (g) / feed intake (g)] X 100.
- Protein efficiency ratio (PER) = Weight gain (g) / protein intake (g).
-Protein productive value (PPV %) = Retained protein (g) / protein intake (g) x 100
-Fat productive value (FPV%) = Retained fat (g) / fat intake (g) x 100
-Condition factor (k) = Body weight (g) / {total length (cm)}3 X 100
- Hepato somatic index (HSI) = Liver weight (g) / fish weight (g) X 100
Physiological measurements: Fish blood samples were collected without anticoagulant. In serum, calorimetric determinations were carried out for total protein and albumin concentrations (using commercial kits);whereas, globulin concentration was obtained by the subtraction of albumin from total protein.
Respiratory burst activity by measuring nitroblutitrazolium activity (NBT): The NBT (yellow) is reduced to formazan (blue) in the reaction with oxygen radicals from neutrophils and monocytes, the analysis of the production of oxygen radicals by the use of NBT can done by spectrofigmeter at 620 nm according to Siwicki et al. (1985).
Lysozyme activity: The lysozyme activity was measured using Figelectric colorimeter with attachment for turbidity measurement. The lysozyme content is determined on the basis of the calibration curve and the extinction measured (Schaperclaus et al. 1992).
Bacterial challenge test: At the end of the experiment, fish at each treatment were randomly divided into two subgroups, each containing 10 fish which were placed into 140-l tanks each. The feeding rate was 5% of biomass per day during 10 days. The challenge experiment was carried out using the strain Aeromonas hydrophila, isolated previously in the laboratory of Fish Disease Department, Central Laboratory for Aquaculture Research, Abbassa, Abo-Hammad, Sharqia, Egypt. Fish were inoculated intraperitoneally (IP) with 0.1 ml bacterial suspension containing 5 x 105 CFU/ml. The same number of fish were inoculated with 0.1 ml saline solution and used as control. Inoculated fish were observed daily for 10 days after inoculation, and mortalities were recorded. These experiments were carried out in duplicate.
Statistical analysis: The obtained data were statistically analyzed using general linear models procedure adapted by SPSS (2004) for user's guide. Least significant difference according to Duncan (1955) within program SPSS was done to determine the degree of significance among means. Data were considered significantly different at P < 0.05.
Results and Discussion
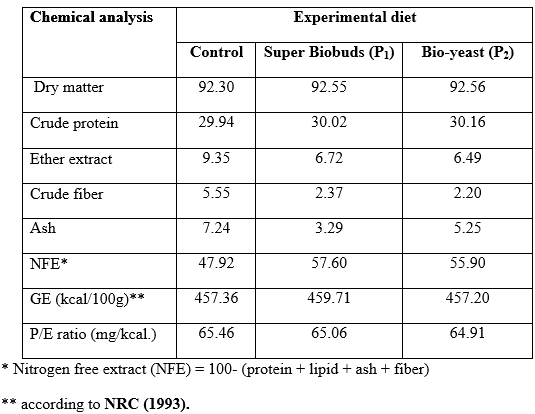
Gain and survival: Table 3 presents the mean values of growth performance parameters of the experimented catfish (Clarias gariepinus) fed either super-Biobuds containing experimental diet (P1) or Bio-yeast containing experimental diet (P2) concerning initial bodyweight (IW), final body weight (FW), weight gain (WG), relative body weight gain (RBWG), average daily gain (ADG), specific growth rate (SGR), and survival rate (SR). The data cleared that probiotics used in the present study caused significant (P≤0.05) improvements in all tested criteria comparing with the controls; although, there were no significant (P≥0.05) differences among the initial body weights. The best FW (36.38 g), WG (20.76 g), SGR (0.93%/d), and SR (100 %) were obtained by using Bio-yeast at 0.1 % of the diet. The improved fish growth may possibly due to the improved feed intake, which may be possibly has happened due to the acceleration of the digestive system development and the increased nutrients digestibility (Suzer et al., 2008).
In this regard, Waché et al. (2006) found that the addition of live yeast improved nutrients digestibility, which may in turn explain the better growth and feed efficiency for all yeast supplements. El-Haroun et al. (2006) examined the probiotic supplement in the diet of Nile tilapia fingerling for 120 days. A probiotic (Biogen®) was used at 0, 0.5, 1.0, 2.0, and 2.5% inclusion rates in the experimental diets. It was found that weight gain and specific growth rate were significantly (P<0.01) higher in the treatment received probiotic (Biogen®) than the control diet, and the optimum Biogen level was 0.5%. El-Haroun (2007) also working on the same probiotic (Biogen®) obtained the same beneficial effects by African catfish.
Table 3: Comparison of growth performance of Clarias gariepinus fed Biobuds (P1) and Bioyeast (P2 ) containing experimental diets (means ± SE).
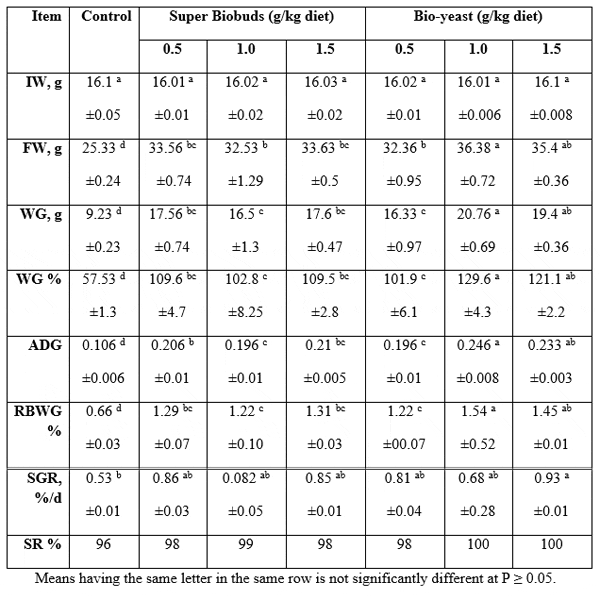
Feed utilization: The following Table 4 presents the data obtained for the experimental fish fed the Super Biobuds and the Bioyeast probiotics containing experimental diets, respectively, concerning feed intake, fat intake, protein intake, feed conversion ratio (FCR), feed efficiency (FE), protein efficiency ratio (PER), protein productive value (PPV), and fat productive value (FPV). The dietary inclusion of the tested probiotics led to significant (P≤0.05) increase in FCR, PER, FER, and FPV besides improving the FCR comparing with the controls. However, there were no significant differences (P≥0.05) among different Super biobuds concentrations. In addition, there were no significant differences (P≥0.05) between 1.0 and 1.5 Bioyeast levels but there were significant (P≤0.05) differences between them and the control. It is noticed that feed intake increased, while FCR decreased significantly (P≤0.05) with the increase of yeast level. The optimum values of these parameters were obtained at 1.0 g yeast/kg diet. These results suggested that yeast supplementation played a role in enhancing feed intake with a subsequent enhancement of the fish body composition. Results of protein productive value (PPV) and fat productive value (FPV) are shown in Table 4. Protein productive value was better in 1.5% Bioyeast (37.2 %) than other treatments. The lowest value of PPV was obtained by control group, but the differences between 0.5 and 0.1% Biobuds were not significant (P > 0.05) in PPV% values (Table 4). Bioyeast (0.1 %) as a probiotics was the best one in its effects on PPV values followed by Biobuds. The best fat productive values (FPV) were obtained by the diet supplemented with 0.1% of Biobuds. The lowest values were obtained by 0.1% Bioyeast and control group.
Table 4: Feed intake, fat intake, protein intake, feed conversion ratio, feed efficiency ratio, protein efficiency ratio, protein productive value and fat productive value of Claris gariepinus fed diets containing different levels of Biobuds (P1) and Bioyeast (P2 ) for 12 weeks (means ± SE).
Similar results were also found by Khalil (1999) who indicated that the dietary inclusion of dried live yeast significantly (P<0.01) improved nutrient utilization (feed conversion, productive value and energy utilization) more than those of the control diets. Abdelhamid et al. (2000) also found that the combination of dried live yeast and lacto-sacc led to significant positive effects on fish feed conversion and nutrient utilization. These results agreed also with Lara-Flores et al. (2003) who evaluated the effect of the supplementation of 0.1% of bacterial mixture a second supplemented at 0.1% with the yeast, S. cerevisiae to diet of Nill tilapia (O. niloticus). They found that fish fed diets supplemented with the yeast showed better feeding efficiency than those fed diets containing the bacterial mixture.
The differences between 0.5 and 1.5 g Biobuds/kg diet and 1 and 1.5 g Bioyeast/kg diet were not significant (P>0.05). In case of Bioyeast and Biobuds, similar results were obtained concerning the effects on the growth performance and feed utilization. However, the most likely explanation of the enhanced feed efficiency and nutrient utilization because of adding a lactobacillus supplement is due to effect in suppressing pathogenic coliform in the nutrients by reducing the thickness of intestinal epithelium (Shelby et al., 2006).
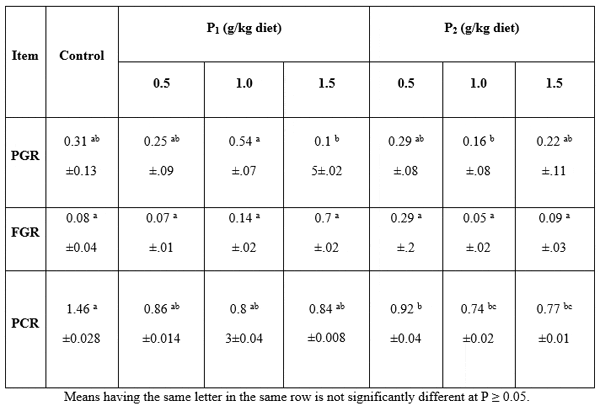
Data presented in Table 5 showed the nutrients growth rate in whole –fish body. Protein growth rate (PGR) was significantly (P>0.05) higher in fish fed Biobuds enriched diets than in that fed the control. Fat growth rate (FGR) showed no significant differences (P>0.05) among all concentrations and control. Protein conversion ratio (PCR) showed no significant differences (P>0.05) between all Biobuds levels but there were significant differences between them and control. However, Abdelhamid et al. (2000; 2002; 2009; 2012 and 2013a, b, c, d) and Mehrim et al. (2013) evaluated many probiotics (yeast, Biogen®, Biomos®, and T-Protphyt 2000®) by Nile tilapia and African catfish and confirmed their beneficial effects on fish performance, feed and nutrient utilization, as well as immunity of fish.
Table 5: Nutrients growth rate in whole body of Clarias gariepinus fed diets containing different levels of Biobuds (P1) and Bioyeast (P2) for 12 weeks (means ± SE).
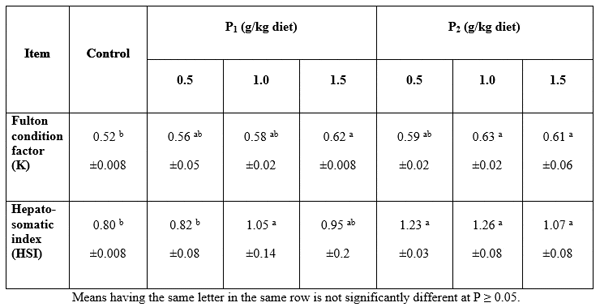
Condition factor: At the end of the experimental period, Fulton condition factor (K), hepato-somatic index (HSI) of catfish fingerlings (Table 6) were significantly affected (P>0.05) by Bioyeast. However, no significant differences were found among different levels of Bioyeast, while there was no significant difference in hepato-somatic index (HSI) among all treatments of Bioyest. However, it significantly differed among different levels of Biobuds. The lowest values of K were obtained by 0.5% Biobuds.
Table 6: Changes in Fulton condition factor (FQ) and hepato somatic index (HSI), of (Claris gariepinus) fed diets containing different levels of Biobuds (P1) and Bioyeast (P2 ) for 12 weeks (means ± SE).
Carcass composition: Data of chemical composition (% DM basis) of fish body at the start and at the end of the experimental period are shown in Table 7 for those fed the Super Biobuds diets and Bio-yeast probiotic diets. It is worthy to note that after feeding trial using commercial probiotics as growth promoters, the carcass contained significantly higher CP compared with the control. The carcass composition concerning DM, CP, and EE contents are increased by age advance, but the ash percentages decreased. At the end of the experiment, DM did not differ significantly (P≥0.05) among all dietary treatments; yet, 1.0 Bioyeast was responsible for the highest CP and the lowest total lipids. These results suggested that due to the high feed intake, nutrient utilization and digestibility, the high changes in protein and lipid content in fish body could be linked with the changes in their synthesis and deposition rate in muscles (Abdel-Tawwab et al., 2008).
Table 7: Proximate chemical analysis (% on dry matter basis) of whole body of (Clarias gariepinus) fed diets containing different levels of Biobuds (P1) and Bioyeast (P2 ) for 12 weeks (means ± SE).
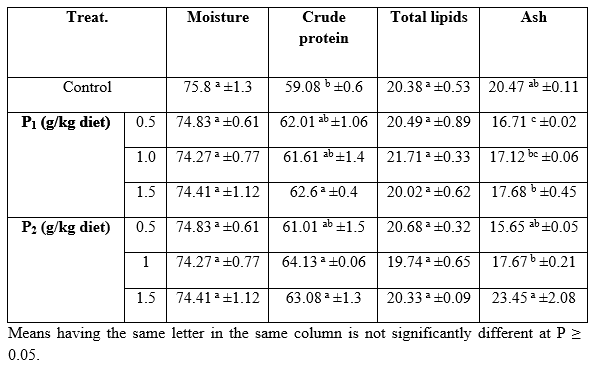
In this regard, Lara-Flores et al. (2003) evaluated the effect of the supplementation of 0.1% of bacterial mixture supplemented at 0.1% with the yeast Saccharomyces cerevisiae to the diet of Nile tilapia. They found that moisture and crude protein were not significantly affected by probiotics, whereas ash content was singnificantly did. El-Haroun et al. (2006) examined the probiotic supplements in the fingerling diet of Nile tilapia, O. niloticus (L) for 120 days. A probiotic (Biogen®) was used at 0, 1.0, 1.5, 2.0 and 2.5%. No significant differences were observed for moisture, protein, and ash contents among the experimental diets, while the lowest gross energy and lipid content were recorded for fish fed the diet containing 0.5% Biogen. However, a negative relationship was noticed between CP and EE contents of fish body but a positive relationship between CP and ash contents was recorded too (Abdelhamid et al., 2007).
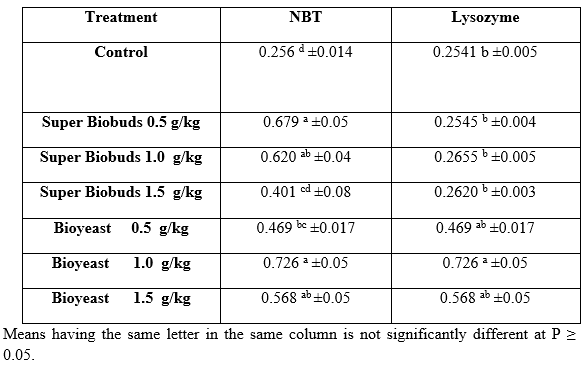
Immunity: Table 8 shows the value of NBT and lysozyme value at the end of the experimental feeding period. There was significant increase in NBT value in the treatments 0.5 and 0.1 P1 as well as 1 and 1.5 P2 compared with the NBT value of the control. There was no significance (P≥0.05) between 0.5 and 1.0 P1 or 1.0 and 1.5 P2. On the other hand, there was no significant difference (P≥0.05) between the lysozyme of control and the other treatments. The results in Table 8 indicated that the value of lysozyme activity was 0.2545 mg/ml serum of the control group and increased significantly (P<0.05) only at 0.5 g/kg of Bioyeast 1.0 g/kg Bioyeast. On the other hand, there were no significant difference between the control and other treatments.
Table 8: Nitro blue tetrazolium activity (NBT, mg/ml) and serum lysozyme activity (µg/ml) in whole body of (Claris gariepinus) fed diets containing different levels of Biobuds and Bioyeast for 12 weeks (means ± SE).
Lysozyme is commonly found in the kidney, spleen, skin mucus, gills, blood plasma, and ovaries of the fish. The enzyme may act as a first defense against bacteria through its presence on external surfaces such as on the skin mucus and gills. These defense strategies, together with the internal function of lysozyme within the major organs, contribute greatly to preventing bacterial infection. Changes in lysozyme activity are common once a fish becomes infected with a pathogen, depending on the level of infection (Siwicki et al., 1998). Moreover, Abdelhamid et al. (2006) detected significant (P<0.05) decrease in lysozyme value in different tissues of fish suffered from various environmental stresses.In the present study, lysozyme level increased in serum of catfish fed diet contained Biobuds as 1.0 or 1.5% compared to fish group fed the control diet. Plasma levels of lysozyme activity were similar between treatments pre and post-challenge with E. ictaluri.
Data in Table 9 showed significant (P<0.05) increase in total protein, albumin and globulin concentration.
Table 9: Serum biochemical changes of Claris gariepinus fed diets containing different levels of Biobuds and Bioyeast for 12 weeks (means ± SE).
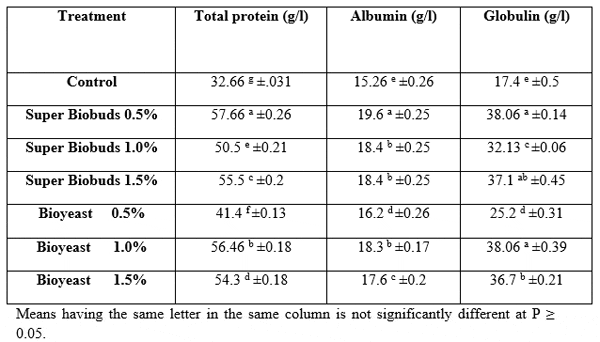
Albumin and globulin concentrations are commonly used for evaluating the effect of nutrients on the fish immunity. In the present study, total protein, albumin, and globulin were significantly enhanced in all experiments compared to control (P<0.05). The increase in these parameters indicates that fish are immunologically strong (Nayak et al., 2007).
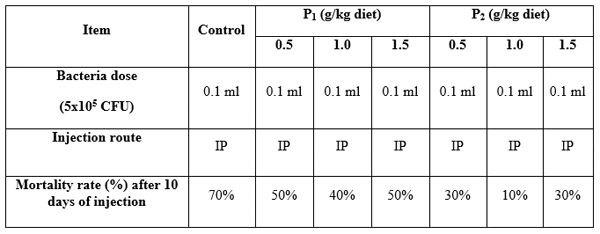
Pathogenicity (against Aeromonas hydrophila): Table 10 shows the fish mortality rate for 10 days after the interperitoneal (IP) injection of Aeromonas hydrophila. The cumulative fish mortality increased significantly by time in all treatments. No fish mortality was observed in all treatments after the 4th day. The total fish mortality after 10 days decreased significantly with the increase of yeast level. The highest mortality after 10 days infection was obtained in the control (70%), whereas the lowest one was obtained for 1.5 g Bioyeast diet (10%). These results suggested that the yeast supplementation could increase the non-specific immune system of fish resulting in fish resistance to A. hydrophila infection. These results agreed with Burgents et al. (2004) who evaluated the effect of yeast supplement on disease resistance in the pacific white shrimp (Litopenaeus vannamei). Animals were fed a standard shrimp pellet diet supplement with 0% (control with 1% grain carrier), 0.5% (with 0.5% carrier) or 1.0% XP for 4 weeks. Fish injected intramuscularly with an LD50 of gram-negative shrimp pathogen, Vibro sp. They found that survival mean of 1.0% XP fed shrimp remained higher than that of the control, but the difference was not significant and their result indicated that the dietary administration of yeast culture could protect shrimp against a decline in resistance to the infection by Vibrio sp. Li and Gatlin (2004) evaluated the use of prebiotics, non-digestible dietary ingredients that beneficially affect the host by selectively stimulating the growth and activating the metabolism of health–promoting bacteria in the intestine tract. Two levels (1% and 2% of diet) of Grobiotic™ AE and brewer's yeast were added to the basal control diet. Each diet was fed to juvenile hybrid striped bass (Morone chrysops x M. saxatilis) for 7 weeks, which exposed to an estimated LD50 of Streptococcus iniae. They found that all groups of fish fed brewer's yeast and Grobiotic™ AE significantly enhanced the survival after both exposure to S. iniae compared to fish fed the basal control diet. Welker et al. (2007) evaluated the effect of dietary supplementation of yeast or yeast subcomponents (YYS) as commercial preparation of β-1,3 glucan, monnan oligosaccharide, or whole-cell Saccharomyces cerevisiae at the manufacturer’s recommended levels on the physiological performance of juvenile channel catfish, I. punctatus for 4 weeks followed by 2 weeks of control diet and measure the effect of dietary β-glucan on resistance to Edwardsiella ictaluri infection. They found that survival from E. ictaluri infection was higher in fish fed YYS diets than in the control group, but the increase were not significant.
Table 10: Mortality rate of Clarias gariepinus fed diets containing different levels of Biobuds (P1) and Bioyeast (P2) and challenged by Aeromonas hydrophila for 10 days.
Aly et al. (2008) evaluated that the potential benefit of Bacillus pumilus and a commercial product (organic green TM) as a probiotic in the culture of Nile tilapia. Two doses of Bacillus pumilus (106 and 1012/g diet) and organic green TM (1 and 2 g/kg diet) were used as feed additives and administered for periods of 1 and 2 months. Group 1 served as a control. Challenge infections were performed after 1, 2 and 8 months using 0.5 ml culture suspension of a pathogenic strain of A. hydrophila (108 bacterial cell/ml). They found that that the potential of using probiotics to enhance immune and health status and to improve disease resistance in Nile tilapia thereby improved growth performance and showed a variable response with the type and dose of treatment, and the period of application.
Rengpipat et al. (2008) isolated lactic acid bacteria (LAB) from adult wild-cought and farmed sea bass (Lates calcorifer) intestines for evaluation as possible probiotics. Fish fed LAB isolates were infected by Aeromonas hydrophila and LC50 of A. hydrophila in aquaria-challenge tests. They found that fish fed on LAB-4 and infected with A. hydrophila at 7 log 10 CFU/ml yielded significantly greater survival compared with control sea bass. El-Boshy et al. (2010) indicated that β- glucan showed significantly increased mortality rate and significantly lower when compared with the control. Moreover, Sakai et al. (2001) reported that the nucleotides from brewer's yeast RNA were capable of enhancing the phagocytic and oxidative activates of kidney phagocytic cells, serum lysozyme in common carp, Cyprinus carpio as well as resistance to A. hydrophila infection. El-Komy and Shehab El-Din (2014) found also that the probiotic organic green culture (Saccharomyces cervisae, Bacillus subtilis, and Aspergillus oryzae), especially at the dose of 1.0 g/kg diet, increased the fish resistance against the pathogenic bacteria Pseudomonas florescence.
Conclusively, it could recommend the usage of 1 g Bioyeast / kg diet of catfish to improve the growth performance and fish quality.
July 2014

