Currently, Pacific white leg shrimp, Litopenaeus vannamei, native to the Pacific coast of Central and South America, is the major shrimp species cultured in China, Taiwan and Thailand (Limsuwan and Chanratchakool, 2004). Since 2010, shrimp farmers in Thailand have experienced the White Feces Syndrome followed by the Early Mortality Syndrome (EMS) in 2012. These diseases caused major economic losses among shrimp farmers throughout the country. Bacterial pathogen especially Vibrio group was reported to be the suspect agents causing mass mortalities (Somboon et al., 2012; Munkongwongsiri et al., 2013).
Many scientists have attempted to solve the problems by using probiotic bacteria or organic acids to reduce the pathogenic bacteria in the gut of shrimp as the prevention methods (Nayak et al., 2012; Walla et al., 2012). Sangrovit WS is one of the natural plant feed additives extracted from plume poppy (Macleaya cordata).
It contains 1.5 per cent sanguinarine, a plant alkaloid which has several pharmacological properties including anti-inflammatory (Chaturvedi et al., 1997; Vrba et al., 2004; Dvorák et al., 2006; Niu et aí.,2012, 2013) and antimicrobial (Nandi et al., 1983; Dzink and Socransky, 1985; Newton et al., 2002; Kosina et al., 2010).
Many studies exhibit the positive effect of Sangrovit on growth performance of animals such as poultry (Vieira et al., 2008a, 2008b), tilapia (Rawling et al., 2009) and sea bass (Korkut et al., 2012). However, their use in shrimp is new. The aim of this study is to evaluate the effect of Sangrovit WS on survival and growth of shrimp. The possibility of Sangrovit WS to prevent Vibrio harveyi infection in shrimp is also evaluated.
Materials and Methods
Experiment 1-The effects of Sangrovit WS on growth and survival of Pacific white shrimp postlarvae
Experimental diets
Three experimental diets were formulated for the present study. Commercial pellet feed without Sangrovit WS was used as control; the other two diets were pellet feed mixed with 100 and 200 mg Sangrovit WS/1 kg of feed. Sangrovit WS for each treatment was applied by dissolving Sangrovit WS powder in distilled water, then spraying and mixing it with commercial pellet feed to obtain a final concentration of 100 and 200 ppm.
Shrimp and experimental protocol
The experiments were carried out at the Aquaculture Business Research Center Laboratory, Faculty of Fisheries, Kasetsart University, Thailand. Postlarvae 9 (PL-9) Pacific white shrimp were obtained from a hatchery in Chachoengsao Province, Thailand. After three days of acclimation, 450 postlarvae (now at PL-12 stage) were randomly distributed into 9 x 500-L fiberglass tanks (three replicate tanks per treatment). Each tank was stocked at a density of 50 PLs per tank to achieve a density of 100 PLs/m2. Each treatment group was fed with the aforementioned diet four times daily for 60 days. Salinity throughout the experiment was maintained at 25 ppt, dissolved oxygen above 4 ppm, and water temperature at 29±1°C.
Growth and survival studies
Ten PLs from each tank were randomly selected and weighed at days 30, 40, 50, and 60 to determine growth. Surviving shrimp were counted on the 60th day and survival rate was calculated using the formula: survival rate ( per cent) = (survival shrimps/total shrimps at 0 day) x 100.
Experiment 2-The effects of Sangrovit WS on growth, survival, and intestinal bacteria of Pacific white shrimps challenged with Vibrio harveyi
Shrimp and experimental protocol
Shrimp from Experiment 1 were fed with the same diet for another 10 days until they reached 10 - 12 g. Then they were randomly distributed into 12 x 500-L fiberglass tanks (four replicate tanks per treatment). Each tank was stocked at a density of 30 shrimp per tank. At the beginning of this experiment (Day 0), Vibrio harveyi was added into each tank to obtain a final concentration of 103 CFU/ml. Each treatment group received the aforementioned diets four times daily for another 30 days. Salinity, dissolved oxygen, and water temperature were maintained as in Experiment 1.
Three tanks from each group were used for growth and survival studies. Another tank from each group was used for intestinal bacterial and histopathological studies.
Growth and survival studies
Ten shrimps from each tank were randomly selected and weighed on days 10, 20, and 30, to determine growth. Surviving shrimps were also counted on these days and survival rate was calculated using the formula: survival rate ( per cent) = (survival shrimps/ total shrimps at 0 day) x 100.
Intestinal bacterial study
Five shrimps from each group were randomly selected and their intestine collected on the days 10, 20, and 30. Intestine of each shrimp was homogenized and spread on TCBS (selective media for Vibrio spp. culture) or NA (general media for most bacteria culture), then incubated at 37°C for 24 hours. All colonies of bacteria were counted and calculated to CFU/g unit.
Histopathological study
Five shrimps from each group were randomly selected and fixed in Davidson's fixative for 48 hours, 30 days after immersion with Vibrio harveyi 103 CFU/ml. All shrimp samples were processed for histopathological study of hepatopancreas and intestine as described by Bell and Lightner (1988).
Statistical analysis
Data were analyzed using the software SPSS v 11.5. One way ANOVA and Duncan's New Multiple Range test were used to compare data among treatments. Differences were considered significant if P<0.05.
Results
Experiment 1-The effects of Sangrovit WS on growth and survival of Pacific white shrimp postlarvae
After 60 days of the feeding trial, shrimp fed with 100 ppm of Sangrovit WS had the highest average body weight of 6.69 ± 0.44 g, followed by 6.60 ± 0.21 g from the group fed with 200 ppm of Sangrovit WS, and 6.49 ± 0.42 g from the control group without Sangrovit WS (Table 1).
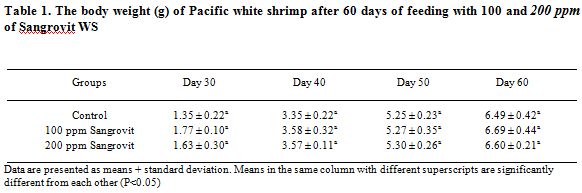
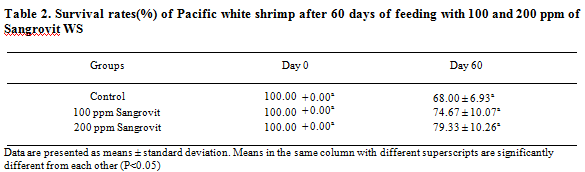
At the end of the feeding trial, shrimp in the group fed with 200 ppm of Sangrovit WS showed the highest average percentage survival rate of 79.33 ± 10.26 per cent, followed by the group fed with 100 ppm of Sangrovit WS which had a 74.67:L 10.07 per cent survival. The average survival rate of these two groups was higher than that of the control group without Sangrovit WS (68.00 ± 6.93 per cent) (Table 2).
Experiment 2-The effects of Sangrovit WS on growth, survival, and intestinal bacteria of Pacific white shrimps challenged with Vibrio harveyi
After 70 days of feeding trial and another 30 days of exposure to 103 CFU/ml of V. harveyi with the same feeding regimen, shrimp given feed mixed with 200 ppm of Sangrovit WS showed the highest average body weight of 16.34 ± 0.78 g which was higher than the groups given feed mixed with 100 ppm of Sangrovit WS (average body weight of 16.10 ± 0.72 g) and the control group (average body weight of 16.16 ± 0.18 g) (Table 3).
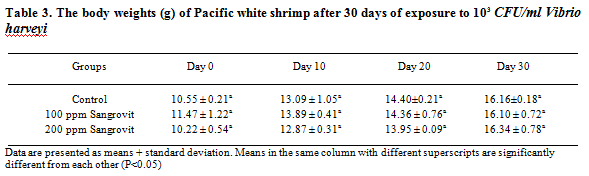
The percentage survival rates of shrimp after the 30 days challenge with V. harveyi at 103 CFU/ml and fed with the same feeding regimen, are presented in Table 4. Shrimp fed 200 ppm of Sangrovit WS had the highest percentage survival rate of 71. 1 1 ± 11.71 per cent, followed by the group fed 100 ppm of Sangrovit WS and the control group which had the average percentage survival rate of 68.89± 10.72 per cent and 66.67±8.82 per cent, respectively.
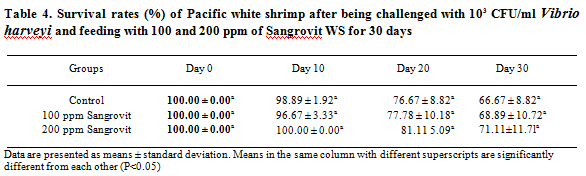
The total number of Vibrio spp. in intestine of experimental shrimp after the 30 days challenge with V harveyi at 103 CFU/ml following the same feeding regimen spp. in as in Experiment 1, is shown in Table 5. Shrimp fed with 200 ppm of Sangrovit WS had the lowest total number of Vibri spp. in intestine throughout the feeding trial. The total number of Vibrio spp. in the intestine of this group was lower than the groups fed with 100 ppm of Sangrovit WS and the control group.
The total number of bacteria in the intestine of shrimp after the 60 days feeding trial (Experiment 1) and 30 days challenge with VV harveyi at 103 CFU/ml (Experiment 2) are shown in Table 6. Shrimp fed with 200 ppm of Sangrovit WS had the lowest total number of bacteria in intestine throughout the experiment. The total number of bacteria in intestine of shrimp in this group was significantly lower than the groups fed with 100 ppm of Sangrovit WS and the control group throughout the feeding trial.
Histopathology of experimental shrimp
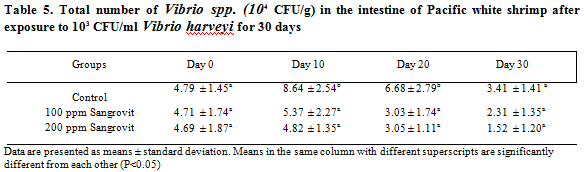
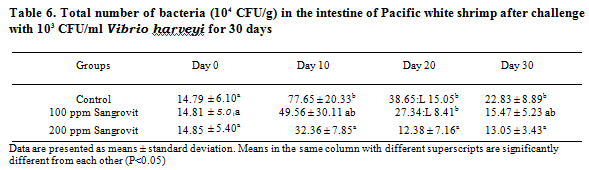
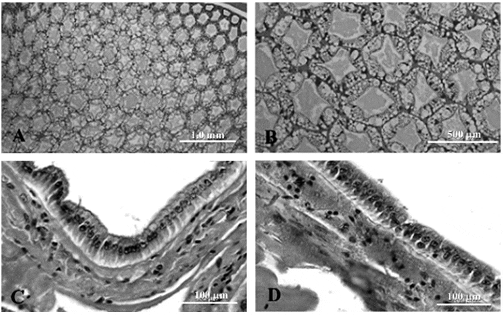
Histopathological study of the hepatopancreas of shrimp revealed signs of mild bacterial infection only in the control group (Figure 7A,7B), while hepatopancreas of shrimp in the group fed with 100 and 200 ppm of Sangrovit WS appeared normal (Figures 8A, 8B, 9A, 9B, respectively). Some shrimp in the control group showed signs of lightly bacterial infection including granulomatous lesions, necrosis of hepatopancreatic cells accompanied by hemocytic infiltration. Whereas the histopathological study of intestines of shrimp from all groups showed no sign of bacterial infection (Figures 7C, 7D, 8C, 8D, 9C, 9D).
Discussion
Despite several studies showing the positive effect of Sangrovit WS on the health of many vertebrate animals (Rawling et al., 2009; Korkut et al., 2012), no such data is available for shrimp.
In the aspect of growth promotion, Sangrovit's active ingredient, sanguinarine, has two important pharmacological properties, namely anti-inflamatory and anti-microbial, similar to those of antibiotic growth promoters (AGPs), especially cycline and macrolide groups, which have been proposed for growth enhancement of AGPs (Dibner and Richards, 2005; Niewold, 2007; Modi et al., 2011). Consequently, the growth-promoting effect of sanguinarine caused by these mechanisms is possible. However, Sangrovit WS showed neither positive nor negative effect on shrimp's growth, determined by body weight, and survival rate in the present study. This may be a consequence of losing some Sangrovit WS from the surface of pelleted feeds due to the high solubility of Sangrovit WS. So the actual dose of Sangrovit WS in shrimp diets may be lower than the expected dose which obscured the growth-promoting effect.
However, the antimicrobial activity of Sangrovit WS in this study was visible. Sangrovit WS could reduce a number of bacterials, both Vibrio spp. and total bacteria, in the intestine of shrimp, especially on days 10 and 20 after being challenged with V harveyi. These results are likely due to the antimicrobial property of sanguinarine alkaloid, by two possible mechanisms of action, namely compromising microbial cytoplasmic membrane (Obiang-Obounou et al., 2011) or perturbing FtsZ assembly dynamics in the Z ring which resulted in bacterial cytokinesis inhibition (Beuria et al., 2005). The ability of Sangrovit to reduce pathogenic bacteria in the intestine of animals was also reported by Gharib Naseri et al. (2012).
The histopathological study showed a slight difference in the appearance of hepatopancreas between the control group and Sangrovit WS-fed groups. The hepatopancreas of shrimp in the control group had a few granulomatous lesions as a result of VV harveyi infection similar to the results obtained by Jiravanichpaisala et al. (1994). However, the normal histopathology of the hepatopancreas of the Sangrovit WS-fed groups could be a results of the anti-inflamatory property of sanguinarine, as reported in other studies (Chaturvedi et al., 1997; Vrba et al., 2004; Dvorák et al., 2006; Niu et al., 2012, 2013). Moreover, the anti-inflammatory effect of Sangrovit in the present study was consistent with a recent study by Vrublová et al., (2010). Meanwhile, the histopathological study of the intestines of shrimp from all groups showed no sign of bacterial infection. This may be because of the low number of V harveyi in the water. The quantities of Vibrio spp. in the intestine of all groups on the final day were not significantly different from each other.
In conclusion, this study revealed that Sangrovit WS could reduce the total number of bacteria in the intestine of shrimp. However, the effect on the growth and survival rate of shrimp was unclear. The benefit of using Sangrovit WS as anti-inflamatory in shrimp or as an alternative to antibiotics for the control pathogenic bacterial infection is promising.
March 2014




