Introduction
Naturally occurring coinfections of pathogens have been reported in various salmonid species. However, the consequences for overall disease resistance remain unclear. Most studies have shown that presence of a primary pathogen reduces the resistance response to a secondary pathogen. For example, in rainbow trout, primary infestation of the parasite Myxobolus cerebralis suppresses the immune system, increasing mortality associated with a secondary infection by Yersinia ruckeri. Similarly, Atlantic salmon infected with IPNV show significantly increased mortality as smolts when exposed to Furunculosis, Vibriosis and ISAv. Conversely, several studies have shown that coinfection does not necessarily increase mortality. For example, some studies on rainbow and brown trout showed that IPNV reduced the infection capacity of the hematopoietic necrosis virus (IHNV) and that the mortality of fish infected with both viruses was significantly lower than that observed in single challenges with each pathogen. Furthermore, acute coinfection of IPNv and ISAv in Atlantic salmon significantly reduced mortality compared with a single infection by ISAv.
Sea lice are among the most important sanitary problems in the global salmon aquaculture industry, further they have been linked to wild salmon and trout population declines around the world. Salmonid infestation by sea lice may be associated with lethal or sub-lethal effects. Sub-lethal effects may include stress, loss of appetite, depression of the immune system and skin damage, and therefore, it can contribute to increased susceptibility to other diseases. In agreement with this hypothesis, Mustafa et al. reported that Atlantic salmon infected with the sea louse Lepeophtheirus salmonis showed increased susceptibility to a microsporidian parasite (Loma salmonae). Recently, Nowak et al., Bustos et al. and Valdes-Donoso et al. suggested, based on field studies on Atlantic salmon, that sea lice affect disease resistance to the amoeba Neoparamoeba perurans and to ISAv.
In Chile, caligidosis caused by Caligus rogercresseyi and piscirickettsiosis caused by Piscirickettsia salmonis have historically been the most important health problems in the salmon industry in the growth-out production phase. C. rogercresseyi is the only sea louse that affects the Chilean salmon industry, and annual losses attributed to this parasite are estimated at more than $178 million US. P. salmonis, an intracellular bacterium, was described in Chile at the end of the 1980s in the X region and was initially found to be strongly associated with Atlantic salmon. It has now extended to other salmonid species farmed in Chile, producing mortality rates of up to 50 per cent in fish in the sea growing stage, with monetary losses exceeding $100 million US per year. Furthermore, P. salmonis has been reported in different countries and can infect rainbow trout (Oncorhynchus mykiss), cherry salmon (Oncorhynchus masou), chinook salmon (Oncorhynchus tshawytscha) and pink salmon (Oncorhynchus gorbuscha).
Resistance of Atlantic salmon to C. rogercresseyi and P. salmonis has recently been studied in single-infection challenges in laboratory conditions by Yañez et al. and Lhorente et al. Lhorente et al. reported heritability of resistance of Atlantic salmon to the sea louse C. rogercresseyi of low (0.03–0.06) and medium (0.22–0.34) magnitudes for the mobile and sessile stages of the parasite, respectively. Other studies performed on different sea lice species confirmed that Atlantic salmon have a heritable defensive mechanism against Lepeophtheirus salmonis and Caligus elongatus. Additionally, it has been shown that both wild and farmed Atlantic salmon have a heritable defensive mechanism against various bacteria, including P. salmonis. For instance, Yañez et al. reported heritability of resistance of Atlantic salmon to P. salmonis ranging from 0.11 to 0.41.
In this study, we hypothesized that coinfection with the sea louse C. rogercresseyi reduces the resistance of Atlantic salmon to P. salmonis because of the well-documented stress and depression of the immune system produced by sea lice infection in salmonids. Additionally, we hypothesized that coinfection resistance is a heritable trait that does not correlate with resistance to a single infection of P. salmonis because salmonid defense mechanisms against bacteria and parasites are substantially different. We present experimental evidence supporting both hypotheses for the interaction between Atlantic salmon, P. salmonis and C. rogercresseyi.
Results
Development of Piscirickettsiosis with and without Coinfection with Sea Lice
The development of piscirickettsiosis was continuously recorded over 53 days until mortality reached 544/544 (100 per cent) and 545/545 (100 per cent) in both coinfection treatments (Figure 1). At that time, mortality in the group that received the single infection with P. salmonis only reached 251/545 (46 per cent). At the beginning of the cohabitation challenge with P. salmonis, a small increase in mortality was observed (day 4). This increase was likely a consequence of the sea lice infection procedure and not a direct consequence of coinfection. At 14–16 days post-infection, mortality associated with piscirickettsiosis was observed in cohabitant fish, and this result was confirmed by PCR at the beginning of the outbreak in dead fish. The daily mortality rate was lower in single than in coinfection scenarios (Figure 1), but no differences were observed between the coinfection treatments.
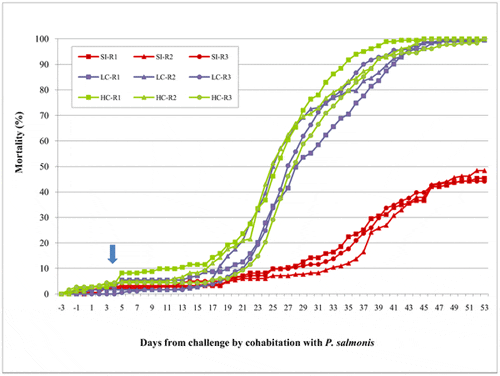
The data show the cumulative mortality of three replicates (tanks) (R1–R3) for three different scenarios: single infection (SI) with P. salmonis and co-infection with two levels of infestation pressure of C. rogercresseyi (low pressure of coinfestation (LC) = 44 copepodites per fish; high pressure of coinfestation (HC) = 88 copepodites per fish). Each replicate had approximately 182 pedigreed fish that were free of disease. The arrow indicates the day of coinfection.
Consistent with the mortality pattern previously described, Kaplan-Meier survival curves (Figure 2) confirmed that coinfection of P. salmonis and C. rogercresseyi significantly (p<0.05) reduced the survival of Atlantic salmon compared to single infection with P. salmonis. However, high parasite burden did not significantly reduce the survival of Atlantic salmon compared to low parasite burden (P>0.05).
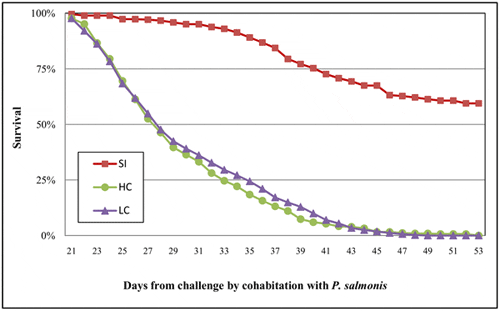
The survival function represents the resistance of the Atlantic salmon (i.e., the proportion that had not died on each day following challenge) in three different treatments: single infection (SI) with P. salmonis and coinfection with two levels of infestation pressure of the sea louse C. rogercresseyi (low pressure of coinfection (LC) = 44 copepodites per fish; high pressure of co infestation (HC) = 88 copepodites per fish).
Genetic Resistance of Atlantic Salmon to Single and Coinfection
As shown in Table 1, all main fixed effects significantly affected the mortality of Atlantic salmon challenged with P. salmonis and coinfected with C. rogercresseyi. The infection effect (I) was highly significant (P<0.001) and showed the highest relative value of the associated sum of squares over the total variability of mortality (28 per cent). The effect of the full-sib family (F) and its interaction with the treatment effect (F×I) were also highly significant (P<0.001). These effects showed a relative influence of 7 per cent and 4 per cent, respectively, on the variability of mortality. Sex and tank within infection treatment effects also significantly affected mortality. However, their contributions to the overall variability of mortality were less than 2 per cent.
Quantitative genetic parameters for the resistance of Atlantic salmon to P. salmonis for the three treatments of single and coinfection are shown in Table 2. The heritability of resistance was very similar between treatments and of medium magnitude (0.17–0.24). Resistance to the single infection with P. salmonis did not correlate phenotypically or genetically with resistance to P. salmonis upon coinfection with the sea louse C. rogercresseyi (Table 2). Conversely, a high and significant genetic correlation for resistance to P. salmonis was observed between the two coinfection treatments (rg LC-HC = 0.99±0.01), providing solid evidence that these resistance values are measurements of the same trait. [For tables 1 and 2, please view the full report]
Discussion
We have demonstrated for the first time that the sea louse C. rogercresseyi, as a secondary pathogen, significantly reduces the resistance of Atlantic salmon to the bacterium P. salmonis. The prevalence of C. rogercresseyi in Chilean salmon farms approaches 100 per cent in some seasons (i.e., spring and summer) and geographic regions. Therefore, its effect on Atlantic salmon mortality may be higher than previously thought. In Atlantic salmon, coinfections of sea lice and other pathogens such as the amoeba Neoparamoeba perurans have also been reported in the USA and in Chilean salmon farms. Both of these studies suggested that sea lice may play an important role in the epidemiology of amoebic gill disease caused by Neoparamoeba perurans and/or in mortality of Atlantic salmon in sea farms. Similarly, Valdes-Donoso et al. reported that most of the ISAV outbreaks between 2007 and 2009 in the Xth region of Chile were associated with high sea lice burdens. The reduced survival upon coinfection in Atlantic salmon might be explained by the direct skin damage caused by parasites that allows other pathogens to enter the fish. Alternatively, it may result from the systemic effects of immunosuppression caused by sea lice.
Genetic variation in resistance to disease in salmonids has been reported for single infections of pathogens in Atlantic salmon, rainbow trout, Coho salmon and brook charr. However, genetic variation in the resistance to coinfection by two pathogens has not been previously estimated in salmonids. Using data from a farmed population of Atlantic salmon, we demonstrated genetic variation for resistance to P. salmonis upon coinfection with the sea louse C. rogercresseyi. Sea lice infections have been reported in salmon farms around the world, but coinfection with bacteria, viruses or parasites has been minimally investigated. Resistance to coinfection has two important implications for salmon breeding. First, if coinfection is common, selection for disease resistance to two or more pathogens, evaluated independently as proposed by Ødegård et al., could be an inefficient method unless resistance to single and coinfection is positively correlated. Second, evaluation of resistance to two different pathogens could be performed in a simple assay, reducing costs associated with laboratory testing. Further studies are necessary to determine whether resistance to coinfection by P. salmonis and C. rogercresseyi or to coinfection by other pathogens relevant to salmon farming such as ISAV, Aeromonas salmonicida or Neoparamoeba perurans occurs in other populations of salmonids.
The genetic correlation for resistance among various Atlantic salmon pathogens has been described for some bacteria and viruses. However, the genetic correlation for resistance between single and coinfection of two pathogens has not been previously estimated in salmonids. Our results strongly suggest that the resistance of Atlantic salmon to a single infection of P. salmonis and that to coinfection with the sea louse C. rogercresseyi are not genetically related. Therefore, we can infer that the best strategy for developing resistance to P. salmonis should consider coinfection with sea lice. However, further studies are necessary to establish whether the resistance to coinfection observed in experimental conditions correlates with higher survival rates in the field. A high genetic correlation for resistance between fresh and sea water has been described for other diseases such as furunculosis, sea lice and IPN.
Conclusion
Infection with the sea louse C. rogercresseyi, as a secondary pathogen, reduces the resistance of Atlantic salmon to the pathogen P. salmonis. Resistance to coinfection of Piscirickettsia salmonis and Caligus rogercresseyi in Atlantic salmon is a heritable trait. The absence of a genetic correlation between the resistance to single infection and that to coinfection indicates that different genes control these processes. Further studies are necessary to investigate the effects of coinfection when the sea louse is the primary pathogen. It is clear that coinfection of different pathogens and resistance to coinfection needs to be considered in future research on salmon farming, selective breeding and conservation.
May 2014


