Identity
Sciaenops ocellatus Linnaeus, 1766 [Sciaenidae]
1766 [Sciaenidae] FAO Names: En - Red drum, Fr - Tambour rouge, Es - Corvinón ocelado
Biological Features
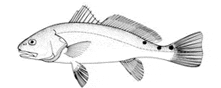
Body elongate with slightly arched back and sloping head. Primarily coppery brown or reddish, though whitish ventrally. Snout blunt with rather large, inferior mouth containing bands of villiform teeth. No barbells, which differentiates this species from closely related black drum (Pogonias cromis). Two dorsal fins, the first with ten hard spines and the second with one hard spine and multiple soft rays (24). Caudal fin slightly concave with one or more large black chromatophores above lateral line. During spawning season, males produce characteristic drumming noise by rubbing specialized muscles against air bladder.
Profile
Historical Background
Interest in the culture of red drum in captivity began in the late 1970s over concerns of declining natural populations due to commercial and recreational fishing activities. Since then a variety of regulatory measures were implemented (e.g. size and bag limits) and commercial fishing has been eliminated entirely in many areas. Initial aquaculture methods were developed in the United States of America, particularly in Texas and Florida, in order to provide alternatives to wild-caught fish and for stock enhancement efforts. With the development of reliable culture techniques, the commercial production of this species has expanded to other countries because red drum is a popular food fish, relatively hardy and adapts well to aquaculture conditions.
Main Producer Countries
Ruditapes decussatus is cultured from the Atlantic coast of France, Spain, Portugal and in the Mediterranean basin.
Habitat and biology
The red drum is a euryhaline fish distributed along the Atlantic and Gulf of Mexico coasts from Cape Cod, Massachusetts to Tuxpan, Mexico. Adults spawn from August through October in coastal waters frequently near tidal inlets. Red drum spawn pelagic eggs (approximately 1.0 mm diameter), with large females producing >1 million eggs. Larvae (approximately 6-8 mm SL) are transported into estuaries via currents where they settle into seagrass meadows. Juveniles and sub-adults typically remain in bays and estuaries moving offshore once they are 3.5-5 years old to join the adult spawning population.
Production
Production Cycle
Production Systems
Seed Supply
Broodstock
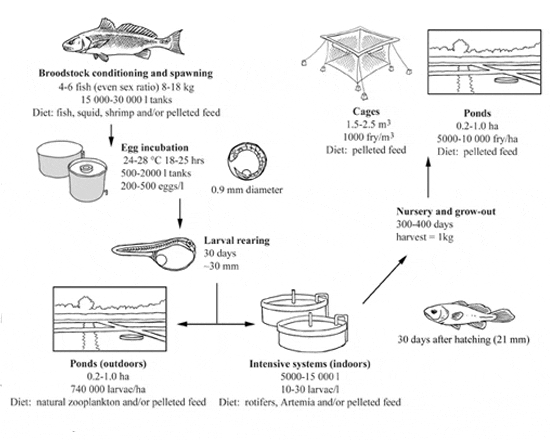
In some cases, eggs and/or fry are purchased from established hatcheries as an alternative to maintaining and spawning broodstock. However, red drum adapt well to captivity and many producers maintain broodstock to ensure a constant supply of larvae for grow-out. Adults can be maintained in circular fibreglass tanks (10 to 17 m3, 1.5 m high) that are connected to an external filter box. Four to six fish may be successfully spawned in such systems using an equal sex ratio. Typically, broodstock are fed 3-5 times a week on a diet of fish, squid, shrimp and/or pelleted feeds.
In the majority of countries producing red drum, broodstock must be imported as it is not an endemic species. As a result, genetic drift is likely to occur over several years of culture with a limited number of broodstock, which may negatively affect production. In order to preserve the genetic diversity of cultured red drum adequate numbers of individuals and families should be maintained in order to minimize inbreeding.
Spawning
Techniques for year-round spawning are well established for this species. A 120 day photoperiod and temperature regime can be used for spawning red drum in captivity. Broodstock are initially acclimated to a temperature of 17 °C and a photoperiod of 9 hours light (HL) which simulates winter conditions for naturally occurring adults. Following the acclimation of the adults, temperature and photoperiod are gradually increased and subsequently decreased over a period of 120 days.
Following initial conditioning of the broodstock, controlled spawning may be achieved throughout the year by manipulations in temperature. For example, spawning may be temporarily suspended when the water temperature drops below 23 °C and resumed once the temperature is raised beyond 23 °C. Red drum usually spawn just after the lights turn off in the evening. The eggs are positively buoyant in salinities greater than 25‰. Overnight, eggs are transported to an external filter box where they are collected in a ~500 micron nitex bag.
Following removal from the egg collector, eggs are set directly into rearing tanks or initially hatched out in incubators (500-2 000 litres; 200-500 eggs/l) and subsequently transferred to the rearing tanks. Disinfection of the eggs with 50-100 ppm formalin for ~1 hr helps decrease bacterial load and fungal growth. Eggs are approximately 1 mm in diameter and hatch within 18-25 hours at temperatures of 24-28 °C.
Hatchery Production
Rearing systems are typically circular fibreglass tanks set in environmentally controlled rooms with average volumes of 5 000-15 000 litres, although first feeding larvae may also be transferred to ponds for culture. Rearing density ranges from 10-30 larvae/l and survival through the larval stage may be >50 percent. Initially, optimal temperature and salinity for hatching and larval rearing are 25-30 °C and 25-30‰, although wider ranges may be used for older larvae. For example, high survival rates have been reported at 10‰, 5‰ and 1‰ for 3 mm, 6 mm and 15 mm larvae, respectively. Larvae may be reared to ongrowing stocking size either within the hatchery or in ponds.
Intensive larval rearing
Red drum larvae are approximately 2.2 mm SL at hatching and are generally ready to feed 3 days post hatch (dph). Larvae are initially fed rotifers at concentrations of 5-10/ml 3-10 dph and subsequently fed Artemia nauplii on 11-15 dph. From this point on, larvae will readily feed on a microparticulate diet, although it is best to co-feed live prey and the inert diet during the weaning period. Alternatively, red drum can be fed a combination of rotifers and inert diet (~0.25 mm) 3-7 dph, followed by inert diet alone - gradually increasing particle size - without sacrificing growth and survival. Live prey are frequently enriched with highly unsaturated fatty acids such as arachidonic acid (ARA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which improve growth and survival. Enrichments are frequently employed to enhance the fatty acid composition of prey items including live microalgae, microalgae paste, commercial preparations and marine fish oils. The addition of microalgae to the rearing tanks (Nannochloris occulata, Isochrysis galbana) at concentrations of 40 000 - 100 000 cells/ml also enhances larval growth and survival. Once fish reach the juvenile stage (less than 1 month), they may be relocated to ponds or net cages for grow-out.
Nursery
Ponds of 0.2-1.0 ha in size are generally filled 5-10 days prior to stocking. Inorganic and organic fertilizers are added to the ponds in order to initiate a phytoplankton bloom that in turn establishes a zooplankton community, primarily consisting of copepods. Larvae (2 dph; 740 000/ha) are moved from the hatchery into ponds once zooplankton levels reach a density of approximately 250 organisms/l. Fish are maintained in these systems through 30 dph (~30 mm SL) and subsequently moved into larger ponds or net cages.
Ongrowing Techniques
Red drum are ongrown in cages or ponds.
Pond culture
In the late 1980s and early 1990s pond culture of red drum in the United States of America was severely affected by the low temperatures encountered during the winter months, which resulted in substantial mortalities. The lower lethal temperature for red drum juveniles is thought to be 8-10 °C, although the expected value depends on salinity and the rate of decline in temperature. In order to combat losses due to low temperatures, culturists have modified production practices. For instance, fish are brought indoors at a weight of approximately 1 g, where they are held in semi-closed recirculating systems, and returned to outdoor ponds (150-225 g) once water temperatures increase. Fingerlings are fairly cannibalistic; frequent grading of the fish while indoors helps to decrease mortality rates, thereby increasing overall production. Market-sized fish (~1 kg) from such systems may be achieved within 11 months.
Cage culture
A variety of cage sizes and types are used for the cage culture of marine finfish. In China, cages are constructed of flexible mesh bags attached to solid frames and are 1.5-2.5 m3 in size. Red drum fingerlings (0.2 g) are stocked at 1 000 fry/m3. As the fish grow, they may be moved into larger cages and the density decreased to ~400 fry/m3. Cannibalism is a frequent problem and the fish are therefore regularly graded to decrease overall mortality. Market size (~1 kg) is normally reached within one year.
Feed supply
Fingerlings grow well on a variety of commercially available feeds formulated specifically for red drum or for other warm water marine species. The nutritional requirements are fairly well understood. Red drum juveniles require a diet containing 35-45 percent protein for maximum growth. Dietary protein is primarily provided by fish meal, although soybean meal may be substituted as long as at least 10 percent of the crude protein is provided by fish meal. As noted above for larvae, juveniles require dietary HUFAs; an inclusion of n-3 HUFAs at ~10 percent of the dietary lipid is recommended. There is limited specific information available regarding the vitamin and mineral requirements of this species; these ingredients are generally added to the diet via commercially available premixes that have been developed for the culture of other fish species. Practical diets for red drum usually contain 40 percent crude protein, 5-7 percent fish oil, <7 percent crude fibre, together with vitamin and mineral premixes.
Harvesting Techniques
Red drum may be harvested from ponds by seine. Multiple harvests from one pond using a grading seine that captures fish of certain sizes results in higher overall production. Alternatively, ponds may be drained and the fish collected in a holding basin from which they are more easily captured. Red drum may be harvested from cages with the use of dip nets following concentration of the fish by slightly raising one side of the bottom net or otherwise lifting the net out of the water.
Handling and Processing
Red drum need careful handling during all stages of culture as well as during harvesting and processing to maintain the nutritional and aesthetic value of the fish, and to avoid contamination. All equipment used to handle or process the fish should be well maintained and thoroughly cleaned before, during and after processing. Local authorities responsible for overseeing the handling and processing of seafood should be contacted for detailed information regarding the sanitary processing of fish.
Production Costs
The following analysis of the production costs associated with operating a red drum aquaculture facility in the United States of America, using ponds for the grow-out of fingerlings from 0.4 g to 1 kg over a time frame of approximately one year, were reported in 1994. Although the reported cost estimations may no longer be valid due to inflation, etc., the factors used to determine the estimates and their relative importance are still relevant today.
| Operating expense | Proportion of annual cost (%) |
|---|---|
| Feed | 55.5 |
| Cost of fingerlings for grow-out | 19.3 |
| Labour | 14.3 |
| Chemicals (e.g. fertilizer, disease treatment) | 4.8 |
| Storage and marketing | 3.4 |
| Equipment maintenance and repair | 2.7 |
Diseases and Control Measures
The following table contains information relating to diseases affecting red drum under culture conditions. The culturist should familiarize themselves with local regulations regarding the use of specific chemicals/medications approved for use in food fish as well as regulations in place for countries importing the product.
| Disease | Agent | Type | Syndrome | Measures |
|---|---|---|---|---|
| Viral nervous necrosis | Nodavirus | Virus | Erratic swimming (spiralling, whirling); neuronal necrosis; swim bladder hyperinflation; darkened colour; reduced feeding | No known treatment; disinfection of culture systems between batches of fish (culture water - UV, ozone; materials - chlorine); culling of sick fish |
| Enteromyxosis (myxidiosis) | Myxidium leei | Myxosporidian endoparasite | Spores in the mucosa of the digestive tract; inflammation of the digestive tract; discoloration; scale loss; skin ulcers | No known treatment; disinfection of culture systems; culling of sick fish |
| Lymphocystis | Iridovirus | Virus | Skin, fins & occasionally gills with enlarged white or pale red dermal fibroblasts (cauliflower-like growths) | No known treatment; disinfection of culture systems; quarantine fish - occasionally the fish will recover on their own; minimize stocking densities |
| Crustacean ectoparasites (sea/fish lice)/td> | Copepods; isopods; branchiurans | External parasites | Small crustaceans attached to gills, mouth, skin; skin ulcers | Fresh water dip (5-15 min); formalin dip (30 min, 2-4 ml formalin in 10 litres) - not approved for food fish |
| Vibriosis (systemic bacterial infection) | Vibrio sp. | Bacterium | Swollen abdomen; skin ulcers; haemorrhages on body; protruded eyeball; lethargy; internal lesions | Antibacterials administered via the water or food; removal & treatment of diseased fish; disinfection of culture systems; reduce stress |
| Amyloodiniosis (marine velvet disease) /td> | Amyloodinium ocellatum | Dinoflagellate | Trophont (appears as small whitish spots) on skin, fins &/or gills; weakness; anorexia; flashing; scratching | Fresh water dip (5-15 min); tank flushing (at least 6 tank volumes per day); filtration (microscreen, drum/ bead filters); copper sulphate - not approved for food fish; formalin dips (30 min, 2-4 ml formalin in 10 litres) - not approved for food fish |
| Cryptocaryonosis (marine white spot disease/marine ich) | Cryptocaryon irritans | Protozoan external parasite | White dusting of the skin; respiratory distress; scratching; sloughing of skin; reduced feeding | Reduced salinity (≤16‰ for ~14 days, ≤10‰ for 3 hours); low temperature <19 °C; copper sulphate - not approved for food fish; formalin dip (30 min, 2-4 ml formalin in 10 litres) - not approved for food fish |
Suppliers of pathology expertise
Generally, governmental agencies, private companies and educational institutes offer services relating to the diagnosis and treatment of diseases affecting aquatic organisms. Assistance in locating diagnostic facilities can be obtained by contacting local authorities that are responsible for overseeing the production of captive fish.
Statistics
Production Statistics
(FAO Fishery Statistic)
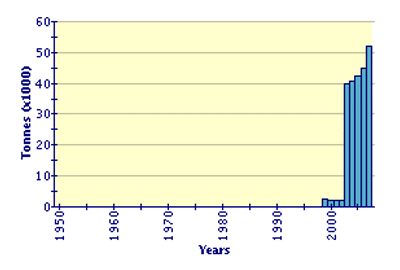
Over 94 percent of the global aquaculture output in 2004 was produced in China. Israel, Mauritius, Mayotte and the United States of America (3 percent) are also significant producers. The total value of global production in 2004 was USD 55 791 000.
Market and Trade
The primary products of the red drum industry are fresh or frozen fillets and steaks (170-340 g), although whole, gutted fish are occasionally sold as well. As with other species, market prices are driven by issues of supply and demand. Currently in the United States, the price of whole red drum purchased from commercial farms is USD 4.19-4.63/kg. No information is currently available on trade of this species in China.
Status and Trends
The red drum is a hardy, relatively fast growing species for which many of the difficulties associated with the culture of marine fishes have been addressed, and in many cases overcome (e.g. spawning, nutrition, environmental tolerances). However, high mortalities due to low water temperatures necessitate careful consideration of production sites and/or systems used by the culturist. Nonetheless, as aquaculture technologies become more advanced it is likely that red drum production will continue to rise worldwide through increased efficiency of existing farms and expansion to additional countries. Further research in the areas of feed efficiency, disease treatment, temperature tolerance and recirculating aquaculture systems will help to ensure the ecologically and economically sound production of this species in coming years.
Main Issues
In areas where red drum are an exotic species, the escape of captive fish from cages and other production containments may negatively affect flora and fauna endemic to the area through competition for resources, such as food and habitat. Another potential problem with the culture of exotic species in the natural environment (i.e. net cages/pen enclosures) is the transfer of diseases to endemic populations; for example it has been suggested that the particular strain of Streptococcus iniae isolated from wild fish (Pomadasys stridens and Synodus variegatus) collected in the Gulf of Eilat (Israel) may have been an exotic strain originating from red drum being reared in nearby cages.
Discharge from land-based operations and uneaten feed/waste products from open aquaculture production systems may negatively impact the surrounding environment. Steps frequently taken to decrease the amount of discharge and/or waste products entering the environment include the treatment of waste water prior to release (filtration, settlement basins), increasing feed efficiency and digestibility, limiting the amount of production in sensitive areas and employing recirculating aquaculture systems for broodstock maintenance, larval culture and/or grow-out operations.
Responsible Aquaculture Practices
Red drum aquaculture should follow the principles outlined in the FAO Code of Conduct for Responsible Fisheries. These practices include maintaining genetic diversity, monitoring fish health, preventing the spread of disease, ensuring a high nutritional value of the product and limiting the environmental impact during culture of the species.
October 2010




